Answers
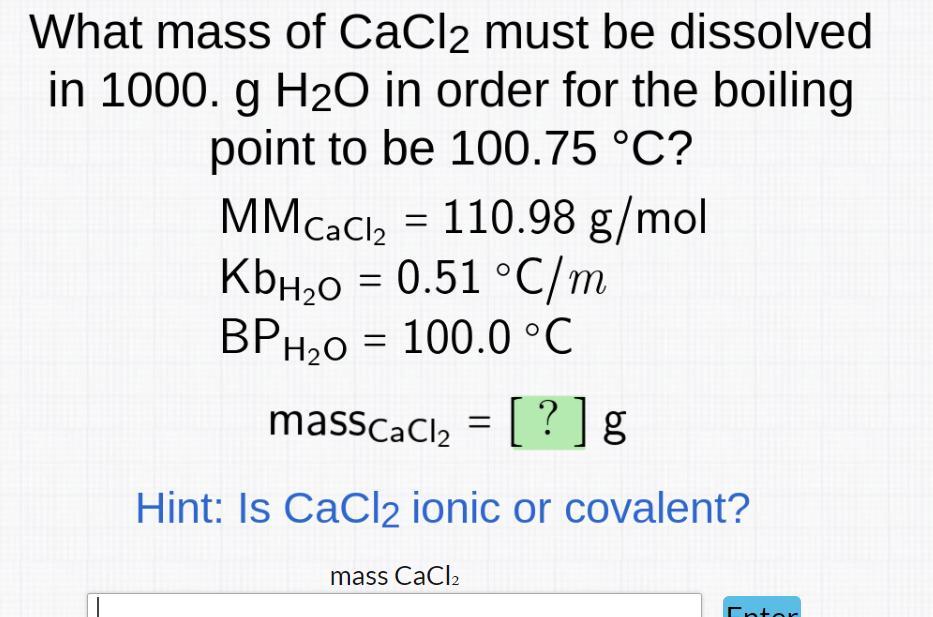
54.2 g of CaCl2 must be dissolved in 1000 g of water to raise the boiling point to 100.75°C.
The mass of CaCl2To solve this problem, we can use the formula:
ΔTb = Kb × m × i
where ΔTb is the boiling point elevation, Kb is the molal boiling point elevation constant for water, m is the molality of the solution, and i is the van't Hoff factor, which represents the number of particles into which the solute dissociates.
We can rearrange this formula to solve for the molality of the solution:
m = ΔTb / (Kb × i)
We know that ΔTb is 0.75°C (100.75°C - 100°C), Kb is 0.51°C/m, and i for CaCl2 is 3 (since it dissociates into 3 ions in water). Substituting these values, we get:
m = 0.75°C / (0.51°C/m × 3) = 0.490 m
To find the mass of CaCl2 needed to make a 0.490 m solution in 1000 g of water, we can use the formula:
moles of solute = molality × mass of solvent (in kg)
We convert 1000 g of water to 1 kg, and then use the molecular weight of CaCl2 to convert from moles to grams:
moles of CaCl2 = 0.490 m × 1 kg = 0.490 mol
mass of CaCl2 = 0.490 mol × 110.98 g/mol = 54.2 g
Therefore, 54.2 g of CaCl2 must be dissolved in 1000 g of water to raise the boiling point to 100.75°C.
Learn more on boiling point here https://brainly.com/question/24675373
#SPJ1
Related Questions
Students are planning to conduct some tests on two substances: candle wax and sulfur crystals
Which property of the wax and the sulfur should be investigated to provide evidence of the relative strengths of their intermolecular forces?
A. Mass
B. Color
C. Texture
D. Melting point
Answers
I did this and I pretty sure it’s A mass cuh
Melting point is the property of the wax and the sulfur should be investigated to provide evidence of the relative strengths of their intermolecular forces. Therefore, the correct option is option D.
What is intermolecular forces?The electrical forces of attraction and repulsion that act between atoms along with other types of nearby particles, such as atoms or ions, are examples of intermolecular forces (IMFs), also known as secondary forces. In comparison to intramolecular forces, which bind a molecule together, intermolecular forces are weak.
For instance, the forces between adjacent molecules are substantially weaker than the covalent bond, which involves sharing pairs of electrons between atoms. Both types of forces are crucial components of the force fields that molecular mechanics typically use. Melting point is the property of the wax and the sulfur should be investigated to provide evidence of the relative strengths of their intermolecular forces.
Therefore, the correct option is option D.
To know more about intermolecular forces, here:
https://brainly.com/question/17111432
#SPJ2
How many grams of Aluminum Sulfate are produced when 4 g of Aluminum Nitrate react with 3 g of Sodium Sulfate?
Al(NO3)3 + Na2SO4 ---------> Al2(SO4)3 + NaNO3
Answers
3.21 grams of Aluminum Sulfate are got when 4 g of Aluminum Nitrate reacts chemcially with 3 g of Sodium Sulfate.
WHat is the balanced equation for this reaction? How many grams of Aluminum Sulfate are produced?The equation given is not balanced. Thus, when balanced the equation becomes:
2 Al(NO₃)₃ + 3 Na₂SO₄ → Al₂(SO₄)₃ + 6 NaNO₃
The molar mass of Al(NO₃)₃ is:
Al(NO₃)₃ = 1(Al) + 3(N) + 9(O) = 213 g/mol
The molar mass of Na₂SO₄ is:
Na₂SO₄ = 2(Na) + 1(S) + 4(O) = 142 g/mol
From the balanced equation, we can see that 2 moles of Al(NO₃)₃ react with 3 moles of Na2SO4 to produce 1 mole of Al₂(SO₄)₃. Therefore, we can calculate the number of moles of Al(NO₃)₃ and Na₂SO₄ that react:
Number of moles of Al(NO₃)₃ = 4 g / 213 g/mol = 0.0188 mol
Number of moles of Na₂SO₄ = 3 g / 142 g/mol = 0.0211 mol
From the balanced equation, we can see that 2 moles of Al(NO₃)₃ produce 1 mole of Al₂(SO₄)₃. Therefore, the number of moles of Al₂(SO₄)₃ produced is:
Number of moles of Al₂(SO₄)₃ = 0.0188 mol / 2 * 1 = 0.0094 mol
The molar mass of Aluminum Sulfate (Al₂(SO₄)₃) is:
Al₂(SO₄)₃ = 2(Al) + 3(S) + 12(O) = 342 g/mol
Therefore, the mass of Aluminum Sulfate produced is:
Mass of Al₂(SO₄)₃ = Number of moles of Al₂(SO₄)₃ * Molar mass of Al₂(SO₄)₃
= 0.0094 mol * 342 g/mol
= 3.21 g
Hence, 3.21 grams of Aluminum Sulfate are liberated when 4 g of Aluminum Nitrate change state with 3 g of Sodium Sulfate.
Learn more about balanced chemical equation here:
https://brainly.com/question/28294176
#SPJ1
The temperature of a 2.0-liter sample of helium gas at STP is increased to 27C, and the pressure is decreased to 80 kPa. What is the new volume of the helium sample? Round your answer to the nearest tenth of a liter?
Answers
The new volume of the helium sample would be 2.4 L.
Volume of a gasAccording to the ideal gas law, PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature in kelvins.
At STP (standard temperature and pressure), which is defined as 0°C (273.15 K) and 101.325 kPa, the volume of 2.0 liters of helium gas contains one mole of helium atoms.
To find the new volume of the helium sample when the temperature is increased to 27°C (300.15 K) and the pressure is decreased to 80 kPa, we can use the following equation:
(P1V1)/T1 = (P2V2)/T2
where P1, V1, and T1 are the initial pressure, volume, and temperature, respectively, and P2, V2, and T2 are the final pressure, volume, and temperature, respectively.
Plugging in the values, we get:
(101.325 kPa)(2.0 L)/(273.15 K) = (80 kPa)(V2)/(300.15 K)
Solving for V2, we get:
V2 = (101.325 kPa)(2.0 L)/(273.15 K) * (300.15 K)/(80 kPa) = 2.36 L
Therefore, the new volume of the helium sample is approximately 2.4 L (rounded to the nearest tenth).
More on gas laws can be found here: https://brainly.com/question/27009857
#SPJ1
how do I convert 0.063 m to centimeters
Answers
Answer:
6.3
Explanation:
multiply the length value by 100
please mark me as brainly listQuestion 4 of 10
How much energy is required to vaporize 2 kg of gold? Use
the table below and this equation: Q = mLvapor
Substance
Aluminum
Copper
Gold
Helium
Lead
Mercury
Water
Latent Heat
Fusion
(melting)
(kJ/kg)
400
207
62.8
5.2
24.5
11.4
335
Melting
Point
(°C)
660
1083
1063
-270
327
-39
0
Latent Heat
Vaporization
(boiling) (kJ/kg)
1100
4730
1720
21
871
296
2256
Boiling
Point
(°C)
2450
2566
2808
-269
1751
357
100
Answers
It requires 10.15 kilojoules of energy.
What is vaporization?The term "vaporisation" (or "evaporation") often refers to the transformation of a liquid's condition into a vapour phase below its boiling point. The phrase, however, can also refer to the process of removing a solvent, independent of the temperature used.
What is energy?When a body moves to exert force, it is said to be exerting work. Energy is the capacity to accomplish work. Energy is something we always need, and it can take many different forms.
If the gold is present in the liquid state, you only have to determine the latent heat of vaporization, or lvap. The empirical data for gold is 330 kJ/mol.
Q = mlvap
Q = (2 kg)(1 kmol/197 kg)(1,000 mol/1 kmol)
Q = 10.15 kJ
It needs an energy of 10.15 kilojoules
To know more about energy visit:
https://brainly.com/question/1932868
#SPJ1
For the equilibrium mixture:
NH4Cl(s) + heat <=> NH4+(aq) + Cl-(aq)
A) What change do you observe when you add concentrated hydrochloric acid, HCl, solution. Give complete explanation.
Answers
The addition of concentrated HCl to the equilibrium mixture will result in the precipitation of more NH₄Cl(s) as the equilibrium shifts towards the left. This can be observed as cloudiness or precipitation forming in the solution.
When concentrated hydrochloric acid (HCl) solution is added to the equilibrium mixture of NH₄Cl(s) + heat <=> NH₄+(aq) + Cl-(aq), the equilibrium will shift towards the left, meaning more solid NH₄Cl will be formed.
This is because HCl is a strong acid that will react with NH₄+ ion to form NH₄Cl(s) and H+ ion:
NH₄+(aq) + Cl-(aq) + HCl(aq) → NH₄Cl(s) + H₂O(l)
The increase in H+ ion concentration due to the addition of HCl will result in the shift of the equilibrium to the left to reduce the excess H+ ion concentration. This will favor the formation of more solid NH₄Cl.
Therefore, the addition of concentrated HCl to the equilibrium mixture will result in the precipitation of more NH₄Cl(s) as the equilibrium shifts towards the left. This can be observed as cloudiness or precipitation forming in the solution.
learn more about equilibrium here
https://brainly.com/question/517289
#SPJ1
The most common nosocomial infection in patients admitted to the hospital?
Rationale: Harding, M., Kwong, J., Roberts, D., Hagler, D., & Reinisch, C. (2020). Lewis’s Medical-surgical nursing : Assessment and management of clinical problems (11th ed.,). Elsevier, Inc.
Answers
Urinary tract infection is the most common nosocomial infection in patients admitted to the hospital. Surgical site wound infections, bacteremia, and gastrointestinal and skin infections are among the most common nosocomial infections.
What is nosocomial infection after hospitalization?A hospital-acquired infection, also known as a nosocomial infection, occurs in a hospital or other healthcare setting. It is sometimes referred to as a healthcare-associated infection to emphasize both hospital and nonhospital settings.
Is a nosocomial infection defined as an infection acquired during a hospital stay?Nosocomial infections, also known as healthcare-associated infections (HAI), are infections acquired while receiving healthcare that was not present at the time of admission.
What is the term for a patient's hospital-acquired infection?Healthcare-Acquired Infections (HAIs), also known as Healthcare-Associated Infections, are infections contracted while receiving treatment at a healthcare facility, such as a hospital, or from a healthcare professional, such as a doctor or nurse.
To know more about nosocomial infection visit:
https://brainly.com/question/30553771
#SPJ1
A headline for a newspaper in a small town reads: "Sheriff Killed by a Poison that has Killed More People Than Any
Other Poison." How was the sheriff poisoned?
thallium
cyanide
arsenic
strychnine
Answers
The sheriff was poisoned by the use of the arsenic poison.
How does arsenic poison work?Arsenic is a toxic substance that can be deadly if ingested or inhaled in high concentrations. It works by disrupting important cellular processes and functions within the body.
When arsenic is ingested, it is absorbed through the digestive system and enters the bloodstream. From there, it is transported to various organs and tissues throughout the body, including the liver, kidneys, and lungs.
Arsenic interferes with the enzymes and proteins that are essential for cellular metabolism, DNA synthesis, and other important cellular processes. This disruption can cause a range of symptoms, including abdominal pain, diarrhea, vomiting, and dehydration.
Arsenic can also cause damage to the nervous system, leading to neurological symptoms such as confusion, seizures, and numbness or tingling in the extremities.
Learn more about arsenic:https://brainly.com/question/493434
#SPJ1
what is the concentration of a 250 mL aqueous solution with 54 grams of KNO3
Answers
Concentration of the 250 mL aqueous solution with 54 grams of KNO₃ is 216 g/L or 216 g/1000 mL.
What is an aqueous solution?An aqueous solution is a solution in which the solvent is water (H₂O). In an aqueous solution, one or more substances, called solutes, dissolve in water to form a homogeneous mixture.
Concentration (in units of g/mL or g/L) = amount of solute / volume of solution
Given the amount of solute (54 grams) and the volume of the solution (250 mL); volume of solution = 250 mL = 0.250 L
So, concentration = 54 g / 0.250 L
concentration = 216 g/L
Therefore, concentration of the 250 mL aqueous solution with 54 grams of KNO₃ is 216 g/L or 216 g/1000 mL.
To know more about aqueous solution, refer
https://brainly.com/question/19587902
#SPJ1
Determine the molarity (M) of 0.2074 g of calcium hydroxide, Ca(OH)₂ (74.09 g/mol), in 40.00 mL of solution.
Answers
Answer:
M=0.06998 mol/L
Explanation:
What is the value of for this aqueous reaction at 298 K?
A+B↽−−⇀C+D
Δ°=17.32 kJ/mol
K= ?
Answers
K has a value of 6.09 105. 6.09 × 10 − 5 . The aqueous reaction for the 298 K reaction is:
The results of substituting the aforementioned variables are: 6.09 10 5.
What exactly are aqueous reactions?Water-based reactions are known as aqueous reactions. It is crucial to comprehend how substances behave in water in order to comprehend them. Some substances are electrolytes; in water, they split into different ions. The behavior of electrolytes varies, though.
How can you tell when a reaction is water-based?
If a problem involves ions or precipitates, you can tell when a solution is aqueous since it has been dissolved in water.
To know more about the aqueous visit:
https://brainly.com/question/31511411
#SPJ1
How much time does it take light to travel 6.03 billion km? (billion=109)
Answer to 3 sig figs.
Answers
Light takes 20,100 seconds or 5.583 hours to travel 6.03 billion km.
How to calculate total time taken using distance and speed?To calculate the time it takes for light to travel 6.03 billion km, we can use the formula:
time = distance / speed of light
where distance is 6.03 x 10^9 km and the speed of light is approximately 299,792,458 meters per second (m/s).
First, we need to convert the distance from kilometers to meters:
distance = 6.03 x 10^9 km x 10^3 m/km = 6.03 x 10^12 m
Now we can calculate the time:
time = distance / speed of light
= 6.03 x 10^12 m / 299,792,458 m/s
= 20,107.394 seconds
To 3 significant figures, the answer is 20,100 seconds or 5.583 hours (since there are 3600 seconds in an hour).
Learn more about light here:
https://brainly.com/question/15200315
#SPJ1
How much energy is involved when 100g of water is heated from 35°C to 115°C water vapor?
Answers
252,212 Joules of energy are required to heat 100g of water from 35°C to 115°C water vapor.
To calculate the amount of energy required to heat water from 35°C to 100°C, we use the specific heat capacity of water, which is 4.18 J/(g°C). This means that it takes 4.18 Joules of energy to heat one gram of water by one degree Celsius.
So, the energy required to heat 100 g of water from 35°C to 100°C can be calculated as follows:
Q1 = m × c × ΔT
Q1 = 100 g × 4.18 J/(g°C) × (100°C - 35°C)
Q1 = 26,212 Joules
Next, we need to calculate the amount of energy required to vaporize the water at 100°C. This is done using the heat of vaporization of water, which is 2260 J/g.
So, the energy required to vaporize 100 g of water at 100°C is:
Q2 = m × Lv
Q2 = 100 g × 2260 J/g
Q2 = 226,000 Joules
Therefore, the total energy required to heat 100 g of water from 35°C to 115°C water vapor is:
Q = Q1 + Q2
Q = 26,212 Joules + 226,000 Joules
Q = 252,212 Joules
Thus, 252,212 Joules of energy are required to heat 100g of water from 35°C to 115°C water vapor.
learn more about energy here
https://brainly.com/question/13881533
#SPJ1
35.0 ml. of a 0.250 M solution of /OH is titrated with 0.150 M HCI. After 35.0 mL of the HCl has been added, the resultant
Answers
Determine the amount of KOH present in the resulting solution. KOH was initially 0.00875 mol, then 0.00525 mol of it interacted with HCl. As a result, 0.00875 mole - 0.00525 mol (= 0.00350 mol of KOH is left. The resulting solution has a volume of 70.0 mL (35.0 mL plus 35.0 mL).
Is HCl directly titrated with NaOH?The titrant (NaOH), which is added gradually throughout the course of a titration, is added to the unknown substance. The equivalency point is the moment at which precisely the right quantity of titrant (NaOH) has indeed been added that react to the entire analyte (HCl).
What happens when you titrate NaOH to HCl?What took place during titration: One mole of NaOH interacts with one mole of HCl inside the reaction between the two substances. NaOH with HCl equals NaCl plus H2O. (NaOH and HCl have a mole ratio of 1:1.) • The NaOH concentration is 0.1 M, or 0.1 molecules per litre.
To know more about solution visit:
https://brainly.com/question/30665317
#SPJ1
Calculate the concentrations of all species in a 0.510 M NaCH3COO (sodium acetate) solution. The ionization constant for acetic acid is a=1.8×10−5.
[Na+]=
[OH−]=
[H3O+]=
[CH3COO−]=
[CH3COOH]=
Answers
The concentrations of all species in a 0.510 M NaCH₃COO (sodium acetate) solution: [Na+]= 0.510 M , [OH-]= 1.8x10⁻⁵ M , [H₃O+]= 1.8x10⁻⁵ M , [CH₃COO-]= 0.510 M and [CH₃COOH]= 0.510 - (1.8x10⁻⁵) = 0.50982 M.
What is concentration?Concentration is the ability to focus your attention on a single task or thought for a prolonged period of time. It involves being able to ignore distractions and to be able to work through any difficulties or obstacles that may arise. Concentration is an important skill to master in order to achieve success in any endeavor, whether it be academic, professional, or personal. Good concentration can help you to stay focused, organized, and productive. When you are able to concentrate, you can take in the information needed to make better decisions and solve problems. Concentration is a skill that can be developed with practice, such as by setting goals, breaking down tasks into smaller, manageable pieces, and avoiding distractions.
To learn more about concentration
https://brainly.com/question/29330415
#SPJ1
Calculate The PH After 15.0 ML Of 0.210 KOH Is Added In The Titration Of 55.0 ML Of .210 M HClOThe Ka Of HClO Is 4.0x10^-8
Answers
The pH after 15.0 mL of 0.210 KOH is added in the titration of 55.0 mL of 0.210 M HClO is 4.56.
To solve this problem, we need to use the balanced chemical equation for the reaction between KOH and HClO:
HClO + KOH → KClO + H2O
We can see that for every mole of KOH added, one mole of HClO will react. Therefore, the number of moles of HClO in 55.0 mL of 0.210 M HClO is:
n(HClO) = M(HClO) x V(HClO) = 0.210 mol/L x 0.0550 L = 0.0116 mol
When 15.0 mL of 0.210 M KOH is added, the number of moles of KOH added is:
n(KOH) = M(KOH) x V(KOH) = 0.210 mol/L x 0.0150 L = 0.00315 mol
Since the reaction is a neutralization reaction, the moles of HClO left after the reaction will be:
n(HClO) = n(HClO)initial - n(KOH) = 0.0116 mol - 0.00315 mol = 0.00845 mol
We can now use the equilibrium expression for the ionization of HClO in water to calculate the pH of the solution:
HClO + H2O ⇌ H3O+ + ClO-
Ka = [H3O+][ClO-]/[HClO]
At equilibrium, the concentrations of H3O+ and ClO- can be assumed to be equal to the concentration of HClO that remains unreacted, since HClO is a weak acid and does not dissociate completely in water. Therefore:
[H3O+] = [ClO-] = [HClO] = 0.00845 mol / (0.0550 L + 0.0150 L) = 0.105 M
Substituting these values into the equilibrium expression for Ka:
Ka = [H3O+][ClO-]/[HClO] = (0.105 M)² / 0.00845 M = 1.31 x 10⁻⁶
pKa = -log(Ka) = -log(1.31 x 10⁻⁶) = 5.88
pH = 1/2(pKw - pKa) = 1/2(14.00 - 5.88) = 4.56
Therefore, the pH after 15.0 mL of 0.210 KOH is added in the titration of 55.0 mL of 0.210 M HClO is 4.56.
To know more about titration, visit:
https://brainly.com/question/31271061
#SPJ1
Which amount of sodium hydroxide is would react exactly with 7.5g of a diprotic acid,H2A(Mr = 150)?
Answers
0.1 mol of sodium hydroxide (NaOH) would react exactly with 7.5 g of the diprotic acid [tex]H_{2}[/tex]A.
What is Molar Mass?
Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). It is calculated by adding up the atomic masses of all the atoms in a molecule or the formula mass of all the ions in an ionic compound.
The balanced chemical equation for the reaction between diprotic acid, [tex]H_{2}[/tex]A, and sodium hydroxide, NaOH, can be represented as follows:
2[tex]H_{2}[/tex]A + 2 NaOH -> [tex]Na_{2}[/tex]A + 2 [tex]H_{2}[/tex]O
From the balanced equation, we can see that 2 moles of [tex]H_{2}[/tex]A react with 2 moles of NaOH to produce 1 mole of [tex]Na_{2}[/tex]A and 2 moles of water ([tex]H_{2}[/tex]O).
First, we need to calculate the number of moles of [tex]H_{2}[/tex]A in 7.5g using the formula:
moles = mass / molar mass
moles of [tex]H_{2}[/tex]A = 7.5g / 150 g/mol = 0.05 mol
Since diprotic acid, [tex]H_{2}[/tex]A, reacts in a 1:2 ratio with NaOH, we need to multiply the moles of [tex]H_{2}[/tex]A by 2 to determine the moles of NaOH required for complete reaction:
Moles of NaOH = 2 * Moles of [tex]H_{2}[/tex]A
Moles of NaOH = 2 * 0.05 mol
Moles of NaOH = 0.1 mol
0.1 mol of sodium hydroxide (NaOH) would react exactly with 7.5 g of the diprotic acid [tex]H_{2}[/tex]A.
Learn more about Molar Mass from the given link
https://brainly.com/question/837939
#SPJ1
CAN SOMEONE HELP WITH THIS QUESTION?
Answers
The percent transmittance (%T) and absorbance (A) of a solution are related by an equation which can be used to solve this question.
What is the absorbance of this solution?The percent transmittance (%T) and absorbance (A) of a mixture are associated by the following equation:
%T = 100 x 10^(-A)
We are given that the %T value of the solution is 51.6% at a wavelength of 550 nm. To find the absorbance (A), we can rearrange the equation above:
A = -㏒(%T / 100)
On substituting the value in the given %T value, we get:
A = -㏒(51.6 / 100) = -㏒(0.516) = 0.286
Therefore, the absorbance of the solution at a wavelength of 550 nm is 0.286.
Learn more about transmittance and absorbance here:
https://brainly.com/question/29705712
#SPJ1
Which sub atomic particles are similar in size
Answers
Answer:
Neutrons and Protons
Explanation:
Different elements can have subatomic particles of varying sizes. The size of an atom is defined by the size of its electron cloud, which is composed of electrons, and the size of its nucleus, which is composed of protons and neutrons. The atomic number and subsequently the identity of an element are determined by the number of protons in the nucleus. The quantity of protons and neutrons in the nucleus determines its size. The quantity of electrons in the electron cloud and the energy levels they are located at define its size. The size of atoms can differ depending on the element due to differences in the amount of protons, neutrons, and electrons.
The volume of a sample of oxygen is 200.0 mL when the pressure is 3.000 atm and the temperature is 37.0 C. What is the new temperature if the volume increases to 400.0 mL and the pressure decreases to 2.000 atm?
Answers
Answer:
140.3 *C
Explanation:
(P1 * V1) / T1 = (P2 * V2) / T2
where P1 = 3.000 atm, V1 = 200.0 ml, T1 = 37.0°C + 273.15 = 310.15 K, P2 = 2.000 atm, V2 = 400.0 ml.
Substituting these values into the formula gives:
(3.000 atm * 200.0 ml) / 310.15 K = (2.000 atm * 400.0 ml) / T2
Solving for T2 gives:
T2 = (2.000 atm * 400.0 ml * 310.15 K) / (3.000 atm * 200.0 ml)
T2 ≈ 413 K or 140°C.
2. Consider the combustion of ethylene,
C₂Ha(g) + 3 O₂(g) → → 2 CO2(g) + 2 H₂O(g)
a)If the concentration of C₂H4 is decreasing at the rate of 0.036 M/s, what are the rates of change
in the concentrations of CO₂ and H₂O?
b) Smol C₂H4 is placed in a 2.0L container, after 1minute, 2mols of C₂H4 remained. What is the
rate of consumption of C₂H4? What is the rate of O₂ in the reaction?
Answers
(a). The rate of change in the concentration of [tex]H_{2}O[/tex] and the rate of change in the concentration of [tex]CO_{2}[/tex] is: 0.072 M/s.
(b). The rate of consumption of [tex]O_{2}[/tex] is: 0.10 mol [tex]O_{2}[/tex] per second.
What is concentration?
a) To determine the rates of change in the concentrations of [tex]CO_{2}[/tex] and [tex]H_{2}O[/tex] , we first need to determine the stoichiometric coefficients of each reactant and product in the balanced chemical equation.
From the balanced chemical equation:
1 mol [tex]C_{2}H_{4}[/tex] reacts to form 2 mol [tex]CO_{2}[/tex] and 2 mol [tex]H_{2}O[/tex].
Therefore, the rate of change in the concentration of [tex]CO_{2}[/tex] is:
(0.036 M/s) x (2 mol [tex]CO_{2}[/tex] /1 mol [tex]C_{2}H_{4}[/tex]) = 0.072 M/s
The rate of change in the concentration of [tex]H_{2}O[/tex] is also:
(0.036 M/s) x (2 mol [tex]H_{2}O[/tex] /1 mol [tex]C_{2}H_{4}[/tex]) = 0.072 M/s
What is consumption?
b) To find the rate of consumption of [tex]C_{2}H_{4}[/tex], we can use the formula:
rate = Δ[ [tex]C_{2}H_{4}[/tex]]/Δt
Initially, the concentration of [tex]C_{2}H_{4}[/tex] is:
n/V = 2 mol / 2.0 L = 1.0 M
After 1 minute, the concentration of [tex]C_{2}H_{4}[/tex] is:
n/V = 2 mol / 2.0 L = 1.0 M
(change in concentration is 0)
Therefore, the rate of consumption of [tex]C_{2}H_{4}[/tex] is:
rate = Δ[ [tex]C_{2}H_{4}[/tex]]/Δt = (1.0 M - 1.0 M) / 60 s = 0 M/s
The rate of [tex]O_{2}[/tex] consumption can be found by using the stoichiometric ratio between [tex]C_{2}H_{4}[/tex] and [tex]O_{2}[/tex] in the balanced chemical equation:
1 mol [tex]C_{2}H_{4}[/tex] reacts with 3 mol [tex]O_{2}[/tex] .
Initially, we have 6 mol [tex]O_{2}[/tex] in the container.
After 1 minute, 2 mol [tex]C_{2}H_{4}[/tex] are consumed, which corresponds to the consumption of 6 mol [tex]O_{2}[/tex] :
6 mol [tex]O_{2}[/tex] / 2 mol [tex]C_{2}H_{4}[/tex] = 3 mol [tex]O_{2}[/tex] / 1 mol [tex]C_{2}H_{4}[/tex]
Therefore, the rate of consumption of [tex]O_{2}[/tex] is:
rate = (3 mol [tex]O_{2}[/tex] / 1 mol [tex]C_{2}H_{4}[/tex]) x (0.0333 mol [tex]C_{2}H_{4}[/tex]/s) = 0.10 mol [tex]O_{2}[/tex] per second.
To know more about consumption rate, visit:
https://brainly.com/question/14269017
#SPJ9
Calculate %m/v composition of 0.022 Kg ammonium nitrate in 587g solution (d=1.07 g/mL)
Answers
[tex]V_{tot} = \frac{587 g}{1,07 g/mL} = 549 mL[/tex]
0,022 kg = 22 g
[tex]\frac{m}{V} = \frac{22 g × 100}{549 mL} = 4,0 % [/tex]
"A certain object's mass is desired to be found after four weighings. If the obtained values are 2.744g, 2.756g, 2.751g, and 2.758g, find the uncertainty in the mass of the object."
Answers
Answer: the uncertainty in the mass of the object is 0.007 g.
Explanation:
The uncertainty in the mass of the object can be calculated using the formula for absolute uncertainty:
Absolute uncertainty = Maximum measured value - Minimum measured value / 2
In this case, the maximum measured value is 2.758 g and the minimum measured value is 2.744 g.
Plugging these values into the formula, we get:
Absolute uncertainty = (2.758 g - 2.744 g) / 2
= 0.014 g / 2
= 0.007 g
So, the uncertainty in the mass of the object is 0.007 g.
if u are satisfy with the answer please vote and rate it thanks
For the reaction: N₂(g) + 3H₂(g) + 2NH3(g) AH = -76.4 KJ/mol. Determine the heat energy when 5.0g of hydrogen burns.
Answers
Answer:
-191 kJ
Explanation:
The given reaction is:
N₂(g) + 3H₂(g) → 2NH₃(g) ΔH = -76.4 kJ/mol
From the balanced equation, we can see that the stoichiometric ratio between hydrogen (H₂) and ammonia (NH₃) is 3:2. This means that 3 moles of hydrogen react to produce 2 moles of ammonia.
To determine the heat energy when 5.0 g of hydrogen (H₂) burns, we need to follow these steps:
Step 1: Calculate the moles of hydrogen (H₂)
Using the molar mass of hydrogen (H₂), which is 2 g/mol, we can calculate the moles of hydrogen (H₂) in 5.0 g of hydrogen:
Moles of H₂ = Mass of H₂ / Molar mass of H₂
Moles of H₂ = 5.0 g / 2 g/mol
Moles of H₂ = 2.5 mol
Step 2: Use the stoichiometry of the reaction
Based on the stoichiometry of the reaction, we know that 3 moles of hydrogen (H₂) react to produce 2 moles of ammonia (NH₃), and the enthalpy change (ΔH) is -76.4 kJ/mol.
Step 3: Calculate the heat energy
The heat energy for 2.5 moles of hydrogen (H₂) can be calculated using the given enthalpy change (ΔH) and the stoichiometry of the reaction:
Heat energy = Moles of H₂ x ΔH
Heat energy = 2.5 mol x -76.4 kJ/mol
Heat energy = -191 kJ (rounded to three significant figures)
So, the heat energy when 5.0 g of hydrogen (H₂) burns is -191 kJ (rounded to three significant figures), and the negative sign indicates that the reaction is exothermic, releasing heat.
1. Ammonia reacts with oxygen to form nitrogen monoxide and
water vapor. How many moles of water are formed when 1.20
moles of ammonia reacts?
Answers
1.8 moles of water are formed when 1.20 moles of ammonia reacts
How is ammonia used?
Ammonia produced by industry is used as fertilizer in agriculture to the tune of 80%. In addition to these uses, ammonia is used to make polymers, explosives, textiles, insecticides, dyes, and other compounds. It is also used to purify water sources.
Ammonia is a colorless, intensely unpleasant gas with a pungent, choke-inducing smell. It readily dissolves in water to produce an ammonium hydroxide solution that can irritate the skin and burn. Ammonia gas is easily compressed and, when put under pressure, turns into a clear, colorless liquid.
4 NH₃ + 5 O₂ → 4 NO + 6 H₂O
4 moles of ammonia gives 6 moles of water
Moles of H₂O = 1.2 moles of NH₃ x 6 moles of H₂O/4 moles of NH₃
Moles of H₂O = 1.8moles
To learn more about ammonia use:
https://brainly.com/question/14854495
#SPJ1
what is the amount of power produced if 35Nm of work is done in 5 seconds
Answers
Answer:
70 watts
Explanation:
Calculate the cell potential, Ecell, for the following reaction at 298k.
Co(s)+2Ag+(0.010M)=Co+2(0.015M)+2 Ag(s)
Answers
To calculate the cell potential, Ecell, for the given reaction at 298K, we need to use the Nernst equation. The Nernst equation relates the cell potential to the standard cell potential, temperature, and the concentrations of the reactants and products. The Nernst equation is given as follows:
Ecell = E°cell - (RT/nF) ln(Q)
where,
Ecell = cell potential
E°cell = standard cell potential
R = gas constant (8.314 J/K.mol)
T = temperature (298 K)
n = number of electrons transferred in the balanced redox reaction
F = Faraday constant (96,485 C/mol)
Q = reaction quotient
The given reaction is a redox reaction, which involves the transfer of two electrons from Co to Ag+. The balanced half-reactions are as follows:
Co(s) → Co2+(aq) + 2 e-
Ag+(aq) + e- → Ag(s)
The standard reduction potentials for these half-reactions are:
Co2+(aq) + 2 e- → Co(s) E°red = -0.28 V
Ag+(aq) + e- → Ag(s) E°red = +0.80 V
The overall standard cell potential can be calculated by subtracting the standard reduction potential of the anode from that of the cathode:
E°cell = E°red,cathode - E°red,anode
= +0.80 V - (-0.28 V)
= +1.08 V
Now we need to calculate the reaction quotient Q using the concentrations of the reactants and products. According to the given information, [Ag+] = 0.010 M and [Co2+] = 0.015 M.
Q = ([Co2+][Ag+]^2)/([Ag+]^2)
= ([0.015][0.010]^2)/([0.010]^2)
= 0.015 M
Substituting the values in the Nernst equation, we get:
Ecell = E°cell - (RT/nF) ln(Q)
= 1.08 - (8.314 x 298 / (2 x 96485)) ln(0.015)
= 0.829 V
Therefore, the cell potential, Ecell, for the given reaction at 298K is 0.829 V.
A balloon ascends at a constant rate V in an atmosphere that is exponentially stratified so that the variation of temperature with altitude is given by T(z) -Toe". The balloon carries a thermocouple temperature sensor having a time constant t. Determine the sensor temperature as a function of time. Sketch the sensor temperature and the actual temperature versus time
Answers
We can plug them into the equation above and plot the temperature of the sensor and the actual temperature against time on a graph to visualize how they change over time during the ascent of the balloon.
What is Temperature?
Temperature is a measure of the average kinetic energy of the particles in a substance, such as a solid, liquid, or gas. It is a scalar quantity that reflects the hotness or coldness of a substance. In other words, temperature indicates how much thermal energy is present in a substance.
This equation describes an exponential decay of the temperature with time. As time goes on, the temperature of the sensor decreases exponentially towards zero.
To sketch the sensor temperature and the actual temperature versus time, we would need additional information, such as the initial temperature T0, the time constant tc, and the rate of ascent V of the balloon.
Learn more about Temperature from the given link
https://brainly.com/question/26866637
#SPJ1
Ascorbic acid ( H2C6H6O2 ) is a diprotic acid with a1=8.00×10−5 and a2=1.60×10−12. Determine the pH of each solution.
A 0.190M ascorbic acid ( H2C6H6O2 ) solution.
pH=
A 0.190M sodium ascorbate ( Na2C6H6O2) solution.
pH=
Answers
Ascorbic acid, also known as Vitamin C, is a water-soluble vitamin that plays an important role in many biological processes in the human body
How do you find out the pH of the given ascorbic acid solution?For the first part
Step 1: Write the dissociation reactions of H₂C₆H₆O₂ in water:
H₂C₆H₆O₂ ⇌ H⁺ + HC₆H₆O²⁻
HC₆H₆O²⁻ ⇌ H⁺ + C₆H₆O₂²⁻
Step 2: Write the equilibrium expressions for each dissociation reaction:
Kₐ₁= [H⁺][HC₆H₆O²⁻ ] / [H₂C₆H₆O₂]
Kₐ₂= [H⁺][C₆H₆O₂²⁻] / [ HC₆H₆O²⁻]
Step 3: Use the given values of Kₐ₁ and Kₐ₂ to set up an ICE table and solve for [H⁺] and pH.
Kₐ₁ = 8.00×10⁻⁵
Kₐ₂ = 1.60×10⁻¹²
[H₂C₆H₆O₂] = 0.190 M
Let x be the concentration of [H⁺] from the dissociation of H₂C₆H₆O₂
[H⁺] = x M
[HC₆H₆O²⁻] = x M
[C₆H₆O₂²⁻] = x(Kₐ₁/Kₐ₂) M
Now, we can substitute the values into the equilibrium expressions to get:
Kₐ₁ = (x)(x) / (0.190-x)
Kₐ₂ = (x)(x(Ka1/Ka2)) / x
Simplifying and solving for x, we get:
x = 7.62 × 10⁻⁴ M
pH = -log[H⁺] = 3.12
Therefore, the pH of a 0.190 M ascorbic acid solution is 3.12.
Learn more about ascorbic acid here:
https://brainly.com/question/28783204
#SPJ1
. Mercury is the only metal which is a liquid at room temperature. The density of mercury is 13.6 g/cm3. What is the mass, in pounds, of 1.00 quart of mercury? [1 liter = 1.057 quart; 1 pound = 453.6 grams]
Answers
According to the question the 1.00 quart of mercury has a mass of 0.0283 lb.
What is mercury?Mercury is the smallest and innermost planet in the Solar System. It is a terrestrial planet with a thin atmosphere composed mostly of oxygen, sodium, and helium. It is one of four rocky planets on the inside of the Solar System, the other three being Venus, Earth and Mars. Mercury is named after the Roman deity Mercury, the messenger of the gods. It is a very dense planet due to its large iron core and its small size.
1 quart = 0.946 liters
1.00 quart of mercury has a mass of 13.6 g/cm³ x 0.946 liters = 12.8 g
To convert the mass of mercury from grams to pounds, divide 12.8 g by 453.6 g/lb.
12.8 g / 453.6 g/lb = 0.0283 lb
Therefore, 1.00 quart of mercury has a mass of 0.0283 lb.
To learn more about mercury
https://brainly.com/question/24257702
#SPJ1