Part B
Calculate the following quantities, and record them in the table:
the number of moles of citric acid used (Use 192.13 grams/mole as the molar mass of citric acid.)
the heat absorbed by the water, in joules (Use Q = mCΔT, where 15.0 milliliters of water has a mass of 15.0 grams. Use 4.186 joules/gram degree Celsius as water’s specific heat capacity.)
the change in internal energy of the mixture of citric acid and sodium bicarbonate. (Assume that energy absorbed by the mixture of citric acid and sodium bicarbonate is released by the water.)
the reaction enthalpy, in joules/mole
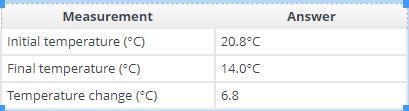
Answers
Recording the answers in the table:
Measurement - AnswerInitial temperature (°C) - 20.8°CFinal temperature (°C) - 14.0°CTemperature change (°C) - 6.8Number of moles of citric acid used - 0.013 molHeat absorbed by the water (J) - 428.3 JChange in internal energy of the mixture (J) - -428.3 JReaction enthalpy (J/mol) - 33,025 J/molHow to calculate measurements?To calculate the number of moles of citric acid used, we need to divide the mass of citric acid used by its molar mass:
Number of moles of citric acid = Mass of citric acid / Molar mass of citric acid
Number of moles of citric acid = (2.50 g) / (192.13 g/mol)
Number of moles of citric acid = 0.013 mol
To calculate the heat absorbed by the water, we can use the formula Q = mCΔT, where Q is the heat absorbed, m is the mass of the water, C is the specific heat capacity of water, and ΔT is the temperature change:
Q = (15.0 g) x (4.186 J/g°C) x (6.8°C)
Q = 428.3 J
To calculate the change in internal energy of the mixture of citric acid and sodium bicarbonate, we can use the fact that the energy absorbed by the mixture is released by the water. Therefore:
ΔU mixture = -Q water = -428.3 J
To calculate the reaction enthalpy, we need to divide the heat absorbed by the number of moles of citric acid used:
Reaction enthalpy = Q / Number of moles of citric acid
Reaction enthalpy = (428.3 J) / (0.013 mol)
Reaction enthalpy = 33,025 J/mol
Find more on temperature change here: https://brainly.com/question/28551912
#SPJ1
Related Questions
explain the relationship among the concentrations of major species in a mixture of weak and strong acids and bases
Answers
The concentrations of major species in a mixture of weak and strong acids and bases are determined by their dissociation behavior and interaction in a solution, influencing the overall pH and buffering capacity.
The relationship among the concentrations of major species in a mixture of weak and strong acids and bases can be understood through their dissociation and interaction in a solution.
Strong acids, such as HCl, fully dissociate in water, releasing a high concentration of H+ ions. Similarly, strong bases, like NaOH, dissociate completely, releasing a high concentration of OH- ions.
Weak acids, such as acetic acid (CH3COOH), only partially dissociate in water, releasing a smaller concentration of H+ ions. Likewise, weak bases, like ammonia (NH3), partially dissociate, releasing a smaller concentration of OH- ions.
When a mixture of weak and strong acids and bases is present, the strong species will react first due to their higher concentrations of H+ or OH- ions. This reaction will affect the pH of the solution, as well as the concentrations of the weak species, as they will be buffered by the strong species.
To learn more about buffering capacity click here
brainly.com/question/24188850
#SPJ11
Calculate the heat capacity, in joules per degree of 28.4 g of water. Specific heat of H2O() = 4.184 J/g.°C a) 28.4 J/°C b) 119 J/°C Oc) 6.8 J/°C d) 0.147J/°C
Answers
The heat capacity of 28.4 g of water is 118.8976 J/°C. The closest option to this answer is option b) 119 J/°C.
To calculate the heat capacity of 28.4 g of water, we need to use the formula:
Heat capacity = mass x specific heat
where mass is given as 28.4 g and specific heat of water is given as 4.184 J/g.°C.
So, substituting the values in the formula, we get:
Heat capacity = 28.4 g x 4.184 J/g.°C
Heat capacity = 118.8976 J/°C
To calculate the heat capacity of 28.4 g of water, you need to multiply the mass of water (m) by its specific heat (c). The formula for heat capacity (Q) is:
Q = m × c
Given:
m = 28.4 g
c = 4.184 J/g.°C
Substitute the values and perform the calculation:
Q = 28.4 g × 4.184 J/g.°C = 118.8 J/°C
The closest answer among the given options is:
b) 119 J/°C
To learn more about mass of water click here
brainly.com/question/26789700
#SPJ11
A Carbon atom has a mass of 1.994 x10-23 g. If a sample of pure carbon has a mass of 42.552g, how many atoms would this contain? Show your work.
Answers
The sample of pure carbon would contain approximately 2.135 x 10²⁴ carbon atoms.
How many carbon atoms have masses that are equivalent to those in the periodic table?The majority of carbon atoms—98.93%—have masses of 12 atomic mass units. A mass of 13.00 atomic mass units is present in 1.07% of the carbon atoms. 14.) Identify one distinction between the nuclei of carbon-12 and carbon-13 atoms in terms of the subatomic particles that can be discovered there.
First, using the atomic mass of carbon, we must determine how many moles of carbon are present in the sample:
1 mole of carbon atoms = 12.01 g of carbon atoms (atomic mass of carbon)
42.552 g of carbon atoms / 12.01 g/mol = 3.545 moles of carbon atoms
Using Avogadro's number, we can then determine how many carbon atoms are present in the sample:
Number of carbon atoms = 3.545 moles of carbon atoms x 6.022 x 10²³ atoms/mole
Number of carbon atoms = 2.135 x 10²⁴ atoms
To know more about carbon atoms visit:-
https://brainly.com/question/30507533
#SPJ1
explain how the gaseous neon atoms in a neon sign emit light
Answers
The gaseous neon atoms in a neon sign emit light when neon atoms gain enough energy to become excited.
At the tube's ends, there is an electrode. Although a neon lamp may operate with either AC (alternating current) or DC (direct current), the glow is only visible around one electrode when DC current is utilised. The majority of neon lights you see operate on AC electricity.
The neon atoms receive enough energy when a 15,000 volt electric voltage is introduced to the terminals to remove one of their outer electrons. Nothing will happen if there is insufficient voltage since there won't be enough kinetic energy for the electrons to break free of their atoms. While unbound electrons are drawn to the positive terminal, positively charged neon atoms (cations) are drawn to the negative terminal. Plasma is the name for these charged particles.
Learn more about Neon atoms:
https://brainly.com/question/1991712
#SPJ4
When an electrical current is applied to the neon gas in the sign, it ionizes the atoms, meaning it strips them of one or more of their electrons.
These newly charged particles then collide with other neon atoms in the tube, transferring some of their energy in the process. As the neon atoms relax back to their ground state, they release this excess energy in the form of light. Specifically, neon emits light in the red-orange range of the visible spectrum, which is why neon signs often have a distinct warm glow.
In summary, the process of ionization and subsequent relaxation of excited neon atoms is what causes a neon sign to emit light.
When a neon sign emits light, the process involves gaseous neon atoms, electron excitation, and the release of photons. An electric current passes through the neon gas, causing the electrons in neon atoms to gain energy and move to a higher energy level (electron excitation).
As these excited electrons return to their original energy level, they release energy in the form of photons, which we perceive as the characteristic glow of a neon sign.
To learn more about ionization click here
brainly.com/question/28385102
#SPJ11
Find the volume of a sample of wood that has a mass of 95. 1 g and a density of 0. 857 g/mL (How do you do this!)
Answers
The volume of the sample of wood is 110.9 mL.
Volume is the measure of the amount of space which is occupied by an object or the substance. It is usually expressed in units such as liters, milliliters, cubic meters, or cubic centimeters. The volume of a solid can be calculated by measuring its dimensions and using mathematical formulas, while the volume of a liquid can be measured directly using a graduated cylinder or a pipette.
To find the volume of the sample of wood, we can apply the following formula;
Density = Mass/Volume
Rearranging the formula, we get;
Volume = Mass/Density
Substituting the given values, we get:
Volume = 95.1 g / 0.857 g/mL
Volume = 110.9 mL
To know more about volume here
https://brainly.com/question/1578538
#SPJ4
A 1.0 liter container is filled with 0.300 M of
PCl5 at 250◦C. The vessel is then held at a
constant temperature until the reaction
PCl5(g) ⇀↽ PCl3(g) + Cl2(g)
comes to equilibrium. It is found that the
vessel contains 0.200 moles of PCl5. What is
the value of the equilibrium constant for the
reaction at this temperature?
Answers
The equilibrium constant (K) for the given reaction at 250°C is 0.200.
What is Equilibrium?
Equilibrium refers to a state of balance or stability in a system where opposing forces or processes are in balance, resulting in no net change over time. In the context of chemical reactions, equilibrium refers to a point at which the rates of the forward and reverse reactions are equal, resulting in a constant concentration of reactants and products over time.
To calculate the equilibrium constant (K) for the given reaction at the given temperature, we can use the concentrations of reactants and products at equilibrium.
Given:
Initial concentration of P[tex]Cl_{5}[/tex] ([P[tex]Cl_{5}[/tex]]0) = 0.300 M
Final concentration of P[tex]Cl_{5}[/tex] ([P[tex]Cl_{5}[/tex]]eq) = 0.200 M
The change in concentration of PCl5 ([PCl5]change) can be calculated as the difference between the initial and final concentrations:
[PCl5]change = [P[tex]Cl_{5}[/tex]]0 - [P[tex]Cl_{5}[/tex]]eq
Substituting the given values into the equation:
[P[tex]Cl_{5}[/tex]]change = 0.300 M - 0.200 M
[P[tex]Cl_{5}[/tex]]change = 0.100 M
According to the balanced chemical equation, the change in concentration of P[tex]Cl_{3}[/tex] and [tex]Cl_{2}[/tex]will also be 0.100 M, as the stoichiometric coefficient of P[tex]Cl_{5}[/tex] in the balanced equation is 1.
Now, we can use the concentrations of reactants and products at equilibrium to calculate the equilibrium constant (K) using the following expression for the given reaction:
K = ([P[tex]Cl_{3}[/tex]]eq * [[tex]Cl_{2}[/tex]]eq) / ([P[tex]Cl_{5}[/tex]eq)
Since the change in concentration of P[tex]Cl_{5}[/tex] is equal to the change in concentration of P[tex]Cl_{3}[/tex] and [tex]Cl_{2}[/tex], we can substitute [P[tex]Cl_{5}[/tex]]change for [P[tex]Cl_{3}[/tex]]eq and [[tex]Cl_{2}[/tex]]eq in the equation:
K = ([P[tex]Cl_{5}[/tex]]change * [P[tex]Cl_{5}[/tex]]change) / [P[tex]Cl_{5}[/tex]]eq
K = (0.200)(0.200) / 0.200
K = 0.200
Using a calculator, we can calculate the value of K:
K = 0.25
Learn more about Equilibrium from the given link
brainly.com/question/18849238
#SPJ1
what, if any, relationship is observed between the most probable molecular speed and the molar mass of the gas? the most probable molecular speed decreases as the molar mass of the gas increases. there is no relationship between the most probable molecular speed and the molar mass. the most probable molecular speed decreases as the molar mass of the gas decreases. the most probable molecular speed increases as the molar mass of the gas increases.
Answers
The correct statement is: the most probable molecular speed decreases as the molar mass of the gas increases. The relationship observed between the most probable molecular speed and the molar mass of the gas is that the most probable molecular speed decreases as the molar mass of the gas increases. This is because heavier molecules have more inertia and therefore move more slowly than lighter molecules. So, the larger the molar mass, the slower the molecular speed.
This relationship can be explained by the equation for the most probable molecular speed (V_p), which is derived from the Maxwell-Boltzmann distribution:
V_p = √(2 * R * T / M)
where:
- V_p is the most probable molecular speed
- R is the ideal gas constant
- T is the temperature in Kelvin
- M is the molar mass of the gas
As you can see from the equation, the most probable molecular speed (V_p) is inversely proportional to the square root of the molar mass (M). This means that when the molar mass increases, the most probable molecular speed decreases, and vice versa.
Learn more about molar mass at https://brainly.com/question/837939
#SPJ11
The relationship observed between the most probable molecular speed and the molar mass of the gas is the most probable molecular speed decreases as the molar mass of the gas increases.
This relationship can be explained by the following steps:
1. Molecular speed refers to the velocity of individual molecules in a gas sample.
2. Molar mass is the mass of one mole of a substance, usually expressed in grams per mole (g/mol).
3. The most probable molecular speed can be estimated using the Maxwell-Boltzmann distribution, which describes the distribution of molecular speeds in a gas.
4. According to this distribution, lighter molecules (with lower molar mass) tend to have higher molecular speeds than heavier molecules (with higher molar mass) at the same temperature.
5. Therefore, as the molar mass of a gas increases, the most probable molecular speed decreases.
To learn more about molecular speed, refer:-
https://brainly.com/question/19243977
#SPJ11
at stp, what is the volume of 4.50 moles of nitrogen gas? at stp, what is the volume of 4.50 moles of nitrogen gas? 101 l 167 l 1230 l 60.7 l 3420 l
Answers
The volume of 4.50 moles of nitrogen gas at STP is approximately 101 L. So, the correct answer is 101 L.
At STP (standard temperature and pressure), the volume of one mole of any gas is 22.4 liters. Therefore, to find the volume of 4.50 moles of nitrogen gas at STP, we can simply multiply the number of moles by the molar volume:
At STP (Standard Temperature and Pressure), the volume of 4.50 moles of nitrogen gas (N2) can be calculated using the ideal gas law:
PV = nRT
Where P is the pressure (which is 1 atm at STP), V is the volume, n is the number of moles, R is the gas constant, and T is the temperature (which is 273.15 K at STP).
Rearranging this equation to solve for V, we get:
V = (nRT)/P
Substituting the values for n, R, P, and T, we get:
V = (4.50 mol x 0.08206 L atm K^-1 mol^-1 x 273.15 K)/1 atm
V = 101.3 L
For such more questions on STP:
https://brainly.com/question/27100414
#SPJ11
what is the total number of joules of heat energy needed to raise the temperature of 10 grams of water from 20 c to 30 c
Answers
The total number of joules of heat energy needed to raise the temperature of 10 grams of water from 20°C to 30°C is 418.4 J. The specific heat capacity of water is 4.184 J/g·°C.
To find the total heat energy needed, we can use the formula:
Q = m·c·ΔT
where:
Q = heat energy (in Joules)
m = mass of the water (in grams)
c = specific heat capacity of water (4.184 J/g·°C)
ΔT = change in temperature (in °C)
Substituting the values given, we get:
Q = 10 g × 4.184 J/g·°C × (30°C - 20°C)
Q = 418.4 J
Therefore, the total number of joules of heat energy needed to raise the temperature of 10 grams of water from 20°C to 30°C is 418.4 J.
Learn more about heat energy
https://brainly.com/question/29210982
#SPJ4
based on the wavelength that the cobalt(ii) chloride solution absorbed most strongly, what color light did the copper(ii) sulfate solution absorb most strongly? green purple orange red
Answers
The color of the light absorbed by the copper (II) sulfate solution cannot be determined solely based on the wavelength absorbed by the cobalt (II) chloride solution.
What wavelength of light was the cobalt II chloride solution most effective at absorbing?The example absorption spectra for cobalt(II) chloride in water is seen below. On the y-axis, a number termed absorbance (which has no units) is shown, and on the x-axis, wavelength (in nanometers). The wavelength at which the absorbance is greatest is 510 nm. This equates to a blue-green colour.
What hue of light can pass through a solution of copper II sulphate?Red light in the spectrum is absorbed by copper(II) ions in solution. All the colours, with the exception of red, will be present in the light that exits the solution. This combination of wavelengths appears to us as a soft blue (cyan).
To know more about chloride solution visit:-
https://brainly.com/question/28792273
#SPJ1
suppose you separate a 2.35 g mixture of sand and salt and recover 1.39 g of salt. what is the percent by mass of salt in the mixture?.
Answers
The percent by mass of salt in the mixture is 59.15%.
To find the percent by mass of salt in the mixture, you need to calculate the total mass of the mixture first.
Total mass of mixture = mass of sand + mass of salt
We know that the total mass of the mixture is 2.35 g and that 1.39 g of salt was recovered. So,
Total mass of mixture = 2.35 g
Mass of salt = 1.39 g
Mass of sand = Total mass of mixture - Mass of salt
Mass of sand = 2.35 g - 1.39 g
Mass of sand = 0.96 g
Now that we know the mass of both salt and sand, we can find the percent by mass of salt in the mixture:
% by mass of salt = (mass of salt / total mass of mixture) x 100
% by mass of salt = (1.39 g / 2.35 g) x 100
% by mass of salt = 59.15%
Therefore, the percent by mass of salt in the mixture is 59.15%.
Know more about mass here:
https://brainly.com/question/86444
#SPJ11
Boyle's Law: Air trapped in a cylinder fitted with a piston occupies 136.5 mL at 1.05 atm pressure. What is the volume of air when the pressure is increased to 1.42 atm by applying force to the piston?
Answers
P1V1 = P2V2
where P1 and V1 are the pressure and volume at the initial state, and P2 and V2 are the pressure and volume at the final state.
We are given:
P1 = 1.05 atm
V1 = 136.5 mL
P2 = 1.42 atm
We can solve for V2:
P1V1 = P2V2
V2 = (P1V1) / P2
V2 = (1.05 atm x 136.5 mL) / 1.42 atm
V2 = 100.9 mL (rounded to one decimal place)
Therefore, the volume of air when the pressure is increased to 1.42 atm is about 100.9 mL.
a 20.0-ml sample of 0.25 m hno3 is titrated with 0.15 m naoh. what is the ph of the solution after 3.2 ml of naoh have been added to the acid? please include two decimal places.
Answers
The pH of the solution after 3.2 mL of NaOH have been added to the HNO3 is 12.33.
To solve this problem, we need to use the equation:
M(acid)V(acid) = M(base)V(base)
Where M is the molarity of the solution and V is the volume in milliliters.
First, we need to calculate the moles of HNO3 in the initial solution:
0.25 M x 20.0 mL = 0.005 moles HNO3
Next, we need to determine how many moles of NaOH were added to the solution:
0.15 M x 3.2 mL = 0.00048 moles NaOH
Since NaOH is a strong base, it will completely react with the HNO3, forming water and a salt. This means that the number of moles of HNO3 is reduced by the number of moles of NaOH:
0.005 moles HNO3 - 0.00048 moles NaOH = 0.00452 moles HNO3 remaining
Now, we can use the equation for the dissociation of HNO3 in water:
HNO3 + H2O → H3O+ + NO3-
The concentration of H3O+ can be found using the equation for the ion product of water:
Kw = [H3O+][OH-]
Kw is a constant equal to 1.0 x 10^-14 at 25°C. At this point, we have added enough NaOH to completely react with the HNO3, which means that all of the H3O+ initially present in the solution has been neutralized.
Therefore, [OH-] = (moles of NaOH added) / (total volume of solution)
[OH-] = 0.00048 moles / (20.0 mL + 3.2 mL) = 0.0214 M
Using Kw, we can calculate [H3O+]:
1.0 x 10^-14 = [H3O+][OH-]
[H3O+] = 4.67 x 10^-13 M
Finally, we can convert this concentration to pH:
pH = -log[H3O+] = -log(4.67 x 10^-13) = 12.33
To learn more about : NaOH
https://brainly.com/question/28504849
#SPJ11
a random copolymer produced by polymerization of vinyl chloride and propylene has a number average molecular weight of 229,500 g/mol and a number degree of polymerization of 4,000. what is the average repeat unit molecular weight? select one: a. 62.5 g/mol b. 42.0 g/mol c. 57.4 g/mol d. 24.0 g/mol
Answers
The average repeat unit molecular weight for average molecular weight of 229,500 g/mol and a number degree of polymerization of 4,000 is equals to the 57.4 g/mol. So, option(c) is right one.
Polymers are large molecules made up of repeating structural units linked together. The degree of polymerization (DP) is the number of repeating units in the polymer molecule. The average molecular weight is the degree of polymerization (MP) multiplied by the molecular weight of the repeat unit (m) is written as [tex] \bar M_n = (DP)(m)[/tex]
We have a random copolymer produced by polymerization of vinyl chloride and propylene.
Average molecular weight= 229500 g/mol
Number degree of polymerization = 4000
Using the above formula, the average repeat unit molecular weight = 229500 g/mol/ 4000
= 57.37 ~ 57.4 g/mol
Hence, required value is 57.4 g/mol.
For more information about degree of polymerization, visit :
https://brainly.com/question/30751495
#SPJ4
How many grams are contained in 2.709 x 10 ^24 atoms of MgCl2?
Answers
rock riddles:i am a rock that was formed when intense pressure folded and warped me. then, i was exposed to extreme heat and i melted. i was ejected from a volcano and i cooled so fast that i dont actually have any visible crystals.what types of rock am i.
Answers
You are an igneous rock, more precisely a volcanic glass or obsidian created by the swift cooling of lava, which lacks any discernible crystals.
What types of rocks are created when a rock is subjected to intense pressure?Metamorphic rocks are produced when rocks are subjected to high pressures, high temperatures, hot mineral-rich fluids, or, more usually, any combination of these circumstances.. These kinds of conditions can be found either deep within the planet or at tectonic plate collisions.
Short note about igneous rock: What is it?Igneous rocks are types of rocks that are formed when molten rock, or rock that has been liquefied by extremely high heat and pressure, cools to a solid condition, according to definitions. When molten rock cools, it solidifies into rocks like basalt, rhyolite, or obsidian. Lava is molten rock that flows out of cracks or vents at volcanic centres.
To know more about obsidian visit:-
https://brainly.com/question/14971262
#SPJ1
describe the relative densities of the phases for most substances. density of gas phase density of liquid phase density of solid phase
Answers
For most substances, the relative densities of the phases are as follows: solid phase > liquid phase > gas phase.
To understand the relative densities of the phases for most substances.
In general, the density of a substance varies depending on its phase (solid, liquid, or gas). Here's a brief description of the relative densities for each phase:
1. Solid phase: In most substances, the solid phase has the highest density. This is because the particles (atoms, molecules, or ions) are tightly packed together in a fixed, organized arrangement, resulting in minimal space between them.
2. Liquid phase: The liquid phase usually has a lower density compared to the solid phase. In this phase, the particles are still close together, but they have more freedom to move around. This increased movement results in a slightly less compact arrangement, thus leading to a lower density.
3. Gas phase: The gas phase has the lowest density among the three phases. In this phase, the particles are widely spaced apart and move freely in all directions. A large amount of empty space between particles contributes to the significantly lower density of the gas phase.
To leran more about relative densities, refer:-
https://brainly.com/question/15164682
#SPJ11
The relative densities of the phases are as follows: solid phase > liquid phase > gas phase.
The relative densities of the phases of most substances vary depending on the specific substance and the conditions it is in. Generally, the solid phase has the highest density, followed by the liquid phase, and then the gas phase. This is because in the solid phase, the molecules are tightly packed together and have little room to move, resulting in a higher density. In the liquid phase, the molecules are still close together but have more room to move around, resulting in a slightly lower density than in the solid phase but a higher density than in the gas phase. In the gas phase, the molecules are more spread out and have the most room to move, resulting in the lowest density of the three phases.
However, it's important to note that some substances may have exceptions to these general trends, depending on their specific molecular structures and the conditions they are in.
To know more about relative density:
https://brainly.com/question/20337365
#SPJ11
which of the following is a true statement regarding entropy? multiple choice question. the entropy of a substance is lowest in the solid phase and highest in the gas phase. the entropy of a system is the same regardless of whether it is in the solid or the gas phase. the entropy of a system is lowest in the gas phase and the highest in the solid phase. the entropy of a system is independent of its phase.
Answers
Answer:
Answer (Detailed Solution Below)
Explanation:
Option 3 : Substance in solid phase has the least entropy.
a fractional distillation involves the use of a fractionating column to provide multiple condensation/evaporation cycles over a given distance. group of answer choices true false
Answers
The given statement "A fractional distillation that involves the use of the fractionating column and to provide the multiple condensation or the evaporation cycles over the given distance" is true as it involves the separation of the miscible liquids.
The Fractional distillation is the type of the distillation that will involves the separation of the miscible liquids. This process will involves the repeated distillations and the condensations. The mixture is separated into the component parts. The separation that happens when the mixture will be heated at the certain temperature and the fractions of the mixture will start to vaporize.
The more will be the volatile components will increase in the vapor state after the heating, and when it is liquefied, the volatile components increase in the liquid state.
To learn more about Fractional distillation here
https://brainly.com/question/27004370
#SPJ4
PLEASE ANSWER 50 POINTS!!!!!
How many grams of NH3 form when 22g H2 react completely?
3H2 + N2 ---> 2NH3
H2: 2 g/mol NH3: 17 g/mol
22g H2 ----> gNH3
Answers
Answer:
mass of NH₃ formed when 22g of H₂ react completely = 124.67 grams
Explanation:
3H₂ + N₂ → 2NH₃
What is stoichiometryThe ratio of coefficients of reactants and products in the above reaction equation (3 : 1 : 2), is known as the stoichiometry of the reaction.
A stoichiometric amount of a reagent is the the optimum amount or ratio where, assuming that the reaction proceeds to completion, all of the reagent is consumed, there is no deficiency of the reagent, and there is no excess of the reagent. Thus if the stoichiometry of a reaction is known, as well as the mass of one of the substances, then it is possible to calculate the mass of any of the other substances.
What is a mole?The mole is a unit of amount of substance established by the International System of Units, to make expressing amounts of reactant or product in a reaction more convenient. As defined by Avogadro's Constant, a mole is 6.022×10²³ amounts of something. The mole is used in stoichiometric calculations, instead of the mass.
Converting between mass and molesTo convert from mass to moles, we need to divide the mass present in grams, by the molar mass of the substance (the sum of the molar masses of the individual elements comprising the compound), in g/mol, to get the moles. This can be represented by the formula: n = m/M, where n = number of moles, m = mass, M = molar mass.
So if we have 22 g of H₂ gas, which reacts completely, and therefore is a stoichiometric amount, then converting this to moles:
n(H₂) = m/M = 22/2 = 11 mol.
Using our stoichiometry, we can see that the ratio of H₂ to NH₃ = 3 : 2.
Therefore, for every 3 moles of H₂ used, we produce 2 moles of NH₃.
n(NH₃) = 2/3 × n(H₂) = 2/3 × 11 = 7.333 mol.
Finally, converting moles back to mass we get:
m(NH₃) = n×M = 7.333×17 = 124.67 grams
∴ mass of NH₃ formed when 22g of H₂ react completely = 124.67 grams
25. j. chadwick discovered the neutron by bombarding with the popular projectile of the day, alpha particles. (a) if one of the reaction products was the then unknown neutron, what was the other product? (b) what is the q-value of this reaction?
Answers
(a) If one of the reaction products was the then unknown neutron, what was the other product is the C -12.
(b) The q-value of this reaction is the 5.9 × 10⁸ J.
The James Chadwick was discovered the neutron during the experiment involving the nuclear reaction in that the beryllium, bombarded with the alpha particles. The equation of the reaction is as :
⁴Be₉ + ²He₄ ----> ⁶C₁₂ + ⁰n₁
(a) If one of the reaction products was the then unknown neutron, what was the other product is the C -12.
(b) The q-value of this reaction is as :
q = mc²
Where,
The m is the mass
The c is the speed of the light.
m = 4.002603 + 2.014102
m = 1.988501
q = 1.988501 × 3 × 10⁸
q = 5.9 × 10⁸ J
To learn more about James Chadwick here
https://brainly.com/question/14559793
#SPJ4
Photoionization processes (e.g., N2 +hν → N2+ + e-) remove UV of <150 nm. Which photoreaction is the principal absorber of UV in the 150-200 nm range in the upper atmosphere?
a) N2 + hv ->2N
b) O2 + hv -> 2O
c) O3 + hv -> O2 + O
d) N2 + O2 + hv -> 2NO
e) NO + O2 + hv -> NO3
Answers
Ozone is the primary absorber of UV radiation in the 150-200 nm range in the upper atmosphere, and its depletion can have significant consequences for life on Earth.
UV radiation with wavelengths between 150-200 nm is highly energetic and can cause damage to living cells by breaking chemical bonds and damaging DNA. Therefore, it is important to prevent most of this radiation from reaching the Earth's surface where it can harm living organisms.
In the upper atmosphere, ozone (O3) plays a crucial role in absorbing this harmful UV radiation through the process of photodissociation. When a molecule of ozone absorbs a photon of UV radiation, it undergoes photodissociation or photolysis, which results in the dissociation of the ozone molecule into an oxygen molecule (O2) and an oxygen atom (O):
O3 + hv -> O2 + O
This process is highly efficient and can absorb more than 97% of the incoming UV radiation in the 150-200 nm range. The oxygen atoms produced in this process can then react with other oxygen molecules to form more ozone, thereby replenishing the ozone layer and continuing this protective cycle.
While other molecules such as nitrogen (N2) and oxygen (O2) can also absorb UV radiation in this range, they are much less efficient at doing so compared to ozone. Therefore, ozone is the primary absorber of UV radiation in the 150-200 nm range in the upper atmosphere, and its depletion can have significant consequences for life on Earth.
Visit to know more about Ozone:-
brainly.com/question/5019112
#SPJ11
What must happen before an animal's cells can use food for energy?
Answers
Answer: broken down into smaller molecules
Explanation: The proteins, lipids, and polysaccharides that make up most of the food we eat must be broken down into smaller molecules before our cells can use them—either as a source of energy or as building blocks for other molecules.
Suppose you add to much water to your kool aid. what do you need to do so that the kook aid will taste the way it’s supposed to? everyone is telling me different things help
Answers
I don't think you can actually do anything since kool aid contains substances that can be easily degradated and usually methods that involves concentration (which is this case since you basically diluted the kool aid with water) are usually quite destructive, especially for sensible substances. I might be wrong, but I don't think you can do anything about this
What is the work required for the separation of air (21-mol-% oxygen and 79-mol-% nitrogen) at 25°C and 1 bar in a steady-flow process into product streams of pure oxygen and nitrogen, also at 25°C and 1 bar, of the thermodynamic efficiency of the process is 5% and if Tσ = 300 K
Answers
Work = 116.1 kJ/mol.
Explanation:
Separating air into pure oxygen and nitrogen requires the removal of one component (nitrogen) from the mixture while leaving the other component (oxygen) behind. This can be accomplished using a cryogenic distillation process, which takes advantage of the different boiling points of the two components.
The thermodynamic efficiency of the process is given as 5%, which means that only 5% of the work input is converted to useful work (i.e., the separation of the components). The remaining 95% is dissipated as waste heat.
The work required for the separation of air can be calculated using the following equation:
W = ΔG / η
where W is the work required, ΔG is the Gibbs free energy change for the separation process, and η is the thermodynamic efficiency.
The Gibbs free energy change for the separation of air into pure oxygen and nitrogen can be calculated using the following equation:
ΔG = RTln(K)
where R is the gas constant, T is the temperature (in kelvin), and K is the equilibrium constant for the reaction. For the separation of air, the equilibrium constant is equal to the ratio of the vapor pressures of nitrogen and oxygen at the given temperature and pressure:
K = P_N2 / P_O2
At 25°C and 1 bar, the vapor pressures of nitrogen and oxygen are:
P_N2 = 0.79 × 1 bar = 0.79 bar
P_O2 = 0.21 × 1 bar = 0.21 bar
Therefore, the equilibrium constant is:
K = 0.79 / 0.21 = 3.76
Substituting this into the equation for ΔG gives:
ΔG = RTln(K) = (8.314 J/mol-K)(298 K)ln(3.76) = -5806 J/mol
The negative sign indicates that the separation process is thermodynamically favorable (i.e., exergonic).
Substituting ΔG and η into the equation for W gives:
W = ΔG / η = (-5806 J/mol) / 0.05 = -116,120 J/mol
The negative sign indicates that work must be done on the system to effect the separation of air. The work required is 116,120 J/mol, or 116.1 kJ/mol.
The value of Tσ = 300 K is not used in this calculation, as it represents the reference temperature for calculating the thermodynamic efficiency.
The value of 300K (or more precisely, Tσ = 298.15 K) is used as the reference temperature for calculating thermodynamic efficiency in some cases, particularly for thermodynamic cycles. However, in the problem given, we are not dealing with a thermodynamic cycle but rather a steady-flow process for the separation of air into its component gases. In this case, the temperature and pressure of the air and product streams are all specified (25°C and 1 bar), and the calculation of the work required for the separation is based on the Gibbs free energy change of the process, which depends on the actual temperature and pressure conditions. Therefore, the value of 300K (or Tσ) is not used in this calculation.
in a binary star system that contains stars with 10 m¤ and 5 m¤, the velocity of the 10 m¤ star will be __________ times the velocity of the 5 m¤ star.
Answers
The velocity of the 10 M¤ star will be 1/2 times the velocity of the 5 M¤ star of binary star system.
In a binary star system, the velocity of each star depends on their masses and distances from each other. According to Kepler's laws, the more massive star will have a smaller orbit radius and a faster orbital velocity. Therefore, in this binary star system with stars of 10 m¤ and 5 m¤, the velocity of the 10 m¤ star will be higher than that of the 5 m¤ star. The exact ratio of their velocities cannot be determined without additional information about their distances and orbits.
In a binary star system, the stars orbit around a common center of mass. According to Kepler's laws of planetary motion, the velocities of the two stars are inversely proportional to their masses.
Let v1 be the velocity of the 10 M¤ star and v2 be the velocity of the 5 M¤ star. Using the inverse proportionality of velocities and masses, we can write the following equation:
v1 / v2 = M2 / M1
where M1 is the mass of the 10 M¤ star and M2 is the mass of the 5 M¤ star. Now, we can plug in the given values:
v1 / v2 = (5 M¤) / (10 M¤)
Simplify the equation:
v1 / v2 = 1 / 2
So, the velocity of the 10 M¤ star will be 1/2 times the velocity of the 5 M¤ star.
Learn more about binary star system here:
https://brainly.com/question/29912300
#SPJ11
The velocity of the 10 m¤ star will be approximately 0.71 times the velocity of the 5 m¤ star in this binary star system.
v = √(GM/r)
[tex]v_10m / v_5m[/tex]= √(G(5m¤) / r) / √(G(10m¤) / r)
Simplifying the equation, we get:
[tex]v_10m / v_5m[/tex] = √(5/10) = √0.5 ≈ 0.71
The star system is a way to represent the electronic configuration of an atom. It is also known as the "Hund's rule star notation" or "star diagram." The star system is used to show the distribution of electrons in different orbitals of an atom. In this notation, each orbital is represented by a circle, and each circle is divided into sections (or lobes) representing the different possible values of the angular momentum quantum number (l).
The sections are labeled using the corresponding values of l, such as s, p, d, f, and so on. Electrons are represented by arrows, with the direction of the arrow indicating the spin of the electron. The arrows are placed in the sections of the orbital circles according to Hund's rule, which states that electrons will fill the orbitals with the same energy level singly and with the same spin before pairing up.
To learn more about Star system visit here:
brainly.com/question/17046229
#SPJ4
uric acid is a weak acid. if the initial concentration of uric acid is 0.110 m and the equilibrium concentration of h3o is 3.4 x 10-2 m, calculate ka for uric acid
Answers
The acid dissociation constant (Ka) for uric acid is [tex]1.0 x 10^-5.[/tex]
The dissociation of uric acid can be represented as follows:
H2UA ⇌ H+ + HUA
The equilibrium expression is given by:
Ka = [H+][HUA-]/[H2UA]
where Ka is the acid dissociation constant, [H+] is the concentration of hydrogen ions, [HUA-] is the concentration of the urate ion, and [H2UA] is the concentration of uric acid.
At equilibrium, the concentration of H2UA is equal to the initial concentration minus the concentration of H+ ions that have been consumed:
[H2UA] = 0.110 - [H+]
The concentration of HUA- can be calculated from the equation:
[HUA-] = [H+]
Substituting the above expressions into the equilibrium expression for Ka, we get
[tex]Ka = ([H+]^2) / (0.110 - [H+])[/tex]
Substituting [H+] = 3.4 x 10^-2 M, we get:
[tex]Ka = [(3.4 x 10^-2)^2] / (0.110 - 3.4 x 10^-2)[/tex]
[tex]Ka = 1.0 x 10^-5[/tex]
Therefore, the acid dissociation constant (Ka) for uric acid is [tex]1.0 x 10^-5.[/tex]
Learn more about dissociation constant
https://brainly.com/question/28197409
#SPJ4
The graph shows the changes in the phase of ice when it is heated. A graph is plotted with temperature in degree Celsius on the y axis and Time in minutes on the x axis. The temperature at time 0 minute is labeled A, the temperature at time 2 minutes is labeled B, the temperature at time 25 minutes is labeled C, the temperature at time 80 is labeled D. Graph consists of five parts consisting of straight lines. The first straight line joins points 0, A and 2, B. The second straight line is a horizontal line joining 2, B and 12, B. Third straight line joins 12, B and 25, C. Fourth straight line is a horizontal line which joins 25, C and 80, C. Fifth straight line joins 78, C and 80, D. Which of the following temperatures describes the value of A?
Answers
We can conclude that the value of A must be less than the value of B. Based on the graph, the value of B is around 0°C. So, we can estimate that the value of A is likely to be around -10°C to 0°C.
What is Temperature?
Temperature is a physical quantity that measures the degree of hotness or coldness of an object or substance. It is a measure of the average kinetic energy of the particles that make up a system.
In simpler terms, temperature is a measure of how fast the atoms and molecules in a substance are moving. When the particles are moving faster, the temperature is higher, and when they are moving slower, the temperature is lower.
Based on the given information, we know that at time 0 minutes, the temperature is labeled as A. Therefore, to find the temperature value of A, we need to look at the y-axis at time 0 minutes.
Since the temperature scale is not given, we cannot determine the numerical value of A directly. However, we can make some observations about the graph to infer the approximate value of A.
Learn more about Temperature from the given link
https://brainly.com/question/26866637
#SPJ1
which pairs of solvents would make good extraction systems? you are currently in a sorting module. turn off browse mode or quick nav, tab to items, space or enter to pick up, tab to move, space or enter to drop. good extraction system poor extraction system
Answers
Immiscible two-component solvent systems containing water, dichloromethane, and diethyl ether are perfect for solvent extraction.
Which of these two solvent combinations cannot be utilised in an extraction procedure?Because they are miscible with water and do not produce a distinct layer, methanol and ethanol are not effective extraction solvents.
What common solvent is employed during the solvent extraction process?A versatile technique that is both easy to use and sensitive is solvent extraction. The evidence container is opened, and a tiny amount of a suitable solvent is introduced (the amount will depend on how much debris is in the container). The most widely used solvent for this procedure is carbon disulfide.
To know more about dichloromethane visit:-
https://brainly.com/question/25413988
#SPJ1
an atomic anion with a charge of has the following electron configuration: 2s22p5what is the chemical symbol for the ion? how many electrons does the ion have?how many electrons are in the ion?
Answers
The chemical symbol for the ion with an atomic anion and a charge of -1, and electron configuration of 2s22p5 is Cl⁻. The Cl⁻ ion has 18 electrons.
This is because the electron configuration matches that of the element chlorine, which is found in group 7 of the periodic table. The Cl⁻ ion is formed when chlorine gains an extra electron to fill its valence shell and achieve a stable octet configuration.
The Cl⁻ ion has 18 electrons in total, as it has gained one extra electron compared to the neutral chlorine atom. The ion now has a full outer shell with 8 electrons, making it stable and less reactive than its neutral counterpart.
The Cl⁻ ion is commonly found in nature, particularly in the form of sodium chloride (NaCl) or table salt. The Cl⁻ ion is also used in various chemical processes, such as in the production of bleach and other disinfectants. Overall, the Cl⁻ ion plays an important role in many chemical reactions and is essential for maintaining the balance of charges in various compounds.
know more about electron configuration here
https://brainly.com/question/29757010#
#SPJ11