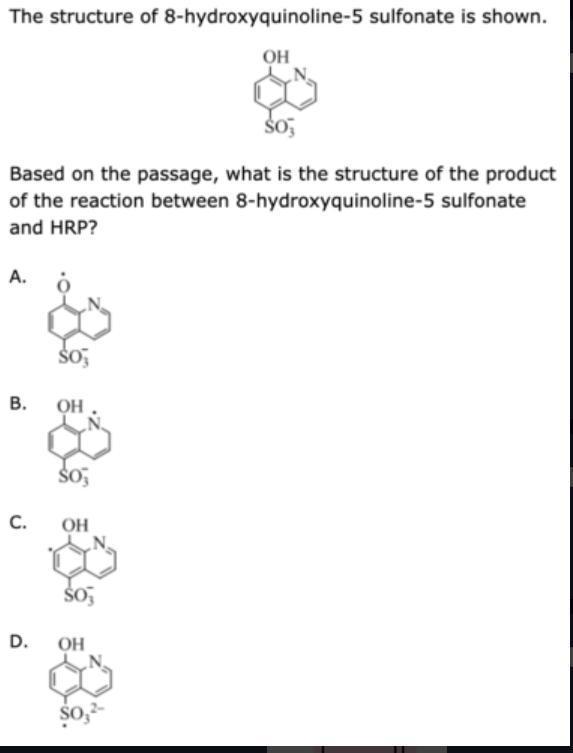
the structure of 8-hydroxyquinoline-5 sulfonate is shown.based on the passage, what is the structure of the product of the reaction between 8-hydroxyquinoline-5 sulfonate and hrp?
Answers
A because the radical produced by the oxidation of an aromatic amine or phenol ring substituent is the end product of this reaction. In this case, the ring substituent is a phenol's hydroxyl group.
8-hydroxyquinoline (8HQ) is a small and planar compound that can chelate metals and have a lipophilic effect. The therapeutic advantages of 8HQ and its derivatives include anti-neurodegenerative, anti-cancer, antioxidant, antibacterial, anti-inflammatory, and anti-diabetic properties.
Occasionally added to phenol-containing organic extraction buffers, 8-hydroxyquinoline inhibits RNase in a partial manner (Kirby, 1956). An antioxidant called 8-hydroxyquinoline keeps phenol stable and stops quinones from developing (phenol oxidation products).
There are numerous applications for hydroxyquinoline sulphate, including topical antiseptics, disinfectants, antiperspirants, deodorants, and fungicides (NTP). It was asked to be used as an antibacterial ingredient in topical salves for cattle (e.g., as an ingredient in bag balm).
To learn more about phenol, refer:
brainly.com/question/19131807
#SPJ4
Related Questions
how many dots would be found in the lewis dot structure for the compound c2h3cl3?
Answers
The number of dots would be found in the Lewis dot structure for the compound [tex]C_{2} H_{3}Cl_{3}[/tex] is 32.
To determine the number of dots in the Lewis dot structure for the compound [tex]C_{2} H_{3} Cl_{3}[/tex] , we first need to know the structure. In the Lewis dot structure, each hydrogen atom has two dots representing two valence electrons and each chlorine atom has six dots representing six valence electrons. The carbon atoms each have four dots representing four valence electrons on their own atoms, and one additional dot on the double bond between them. Therefore, the total number of dots in the Lewis dot structure for the compound [tex]C_{2} H_{3} Cl_{3}[/tex] is:
(2 x 4) + (3 x 2) + (3 x 6) = 8 + 6 + 18 = 32
Learn more about Lewis dot structure here :
https://brainly.com/question/20300458
#SPJ11
There would be 32 dots in the Lewis dot structure for the compound [tex]C_{2}H_{3}Cl_{3}[/tex].
How to determine the number of dots in a compound?To determine the number of dots in the Lewis dot structure for the compound [tex]C_{2}H_{3}Cl_{3}[/tex]., we need to calculate the total number of valence electrons for each element in the compound.
1. Identify the number of valence electrons for each element:
- Carbon (C) has 4 valence electrons.
- Hydrogen (H) has 1 valence electron.
- Chlorine (Cl) has 7 valence electrons.
2. Calculate the total number of valence electrons in the compound:
- There are 2 carbon atoms, so 2 * 4 = 8 valence electrons for carbon.
- There are 3 hydrogen atoms, so 3 * 1 = 3 valence electrons for hydrogen.
- There are 3 chlorine atoms, so 3 * 7 = 21 valence electrons for chlorine.
3. Add up the total number of valence electrons:
- 8 (from carbon) + 3 (from hydrogen) + 21 (from chlorine) = 32 valence electrons.
To know more about Lewis Structures:
https://brainly.com/question/12792895
#SPJ11
The temperature of a sample of gas is 350K at 2.5 atm and 45.0 L. What is the new volume at standard temperature and pressure?
Answers
Answer:
The answer for V2 is 144 to the nearest whole number
Explanation:
P1V1/T1 = P2V2/T2.
P1=2.5atm
V1=45L
T1=350K
P2=1 atm at standard pressure
V2=?
T2=273 at standard temperature
P1V1/T1 = P2V2/T2.
V2=P1V1T2/P2T1
V2=2.5×45×350/1×273
V2=144.23
V2=144 to the nearest whole number
b. i. instead of conc nh, being added to the test solution, 6 m naoh is added (both are bases). how will this affect the separation of the fe?* from the ni? ions in the test solution? explain.
Answers
Using 6 M NaOH instead of concentrated [tex]NH_{3}[/tex] in the test solution will not effectively separate the [tex]Fe^{3+}[/tex] and [tex]Ni^{2+}[/tex] ions because both Ions will form insoluble hydroxides that precipitate from the solution. Concentrated [tex]NH_{3}[/tex]is preferred because it forms complex ions with different solubilities, allowing for the separation of the two ions.
The effect of 6 M NaOH on the separation of [tex]Fe^{3+}[/tex] and [tex]Ni^{2+}[/tex] ions in the test solution instead of concentrated [tex]NH_{3}[/tex]
When using concentrated [tex]NH_{3}[/tex] as the base in the test solution, the [tex]Fe^{3+}[/tex] ions react with [tex]NH_{3}[/tex] to form a complex ion, [tex][Fe(NH_{3} )_{6} ]^{2+}[/tex], while the [tex]Ni^{2+}[/tex] ions form a complex ion,[tex][Ni(NH_{3} )_{6} ]^{2+}[/tex]. These complex ions have different solubilities in the solution, allowing for the separation of [tex]Fe^{3+}[/tex] and [tex]Ni^{2+}[/tex] ions.
However, when using 6 M NaOH as the base, both[tex]Fe^{3+}[/tex] and [tex]Ni^{2+}[/tex] ions will react with the hydroxide ions [tex]OH^{-}[/tex] to form their respective insoluble hydroxides: [tex]Fe(OH)_{3}[/tex] and [tex]Ni(OH)_{2}[/tex]. Both hydroxides will precipitate out of the solution, making it difficult to separate the [tex]Fe^{3+}[/tex] and [tex]Ni^{2+}[/tex] ions.
For More Such Questions on Ions
https://brainly.com/question/1310794
#SPJ11
Write the formulas for the following compounds:a. mercury(II) nitrateb. ammonium phosphatec. calcium silicated. lead(II) chromate
Answers
Formulas of the following compounds are:
mercury (ii) nitrate is [tex]Hg(NO_3)_2[/tex]
ammonium phosphate is [tex](NH_4)_3PO_4[/tex]
calcium silicate is [tex]CaSiO_3[/tex]
lead(II) chromate is [tex]PbCrO_4[/tex]
The formulas of the compound are created by writing the ions of the compound. Then the charge on each ion is crossed with each other and becomes their subscript.
Thus, one can write the formula of mercury (ii) nitrate as
ions = [tex]Hg^{2+[/tex] and [tex]NO_3^-[/tex]
cross the valency of both that is 2 and 1
thus we can write the formula as [tex]Hg(NO_3)_2[/tex]
One can write the formula of ammonium phosphate as
ions = [tex]NH_4^+[/tex] and [tex]PO_4^{3-[/tex]
cross the valency of both that is 1 and 3
thus we can write the formula as [tex](NH_4)_3PO_4[/tex]
One can write the formula of calcium silicate as
ions = [tex]Ca^{2+[/tex] and [tex]SiO_3^{2-[/tex]
cross the valency of both that is 2 and 2. These valencies cross each other out and the subscript is 1 each
thus we can write the formula as [tex]CaSiO_3[/tex]
One can write the formula of lead(II) chromate as
ions = [tex]Pb^{2+[/tex] and [tex]CrO_4^{2-[/tex]
cross the valency of both that is 2 and 2. These valencies cross each other out and the subscript is 1 each
thus we can write the formula as [tex]PbCrO_4[/tex]
Learn more about Chemical formulas:
https://brainly.com/question/11574373
#SPJ4
what is the voltage of a galvanic cell that does 788 j of work when 255 coulomb of charge is transferred?
Answers
The voltage of the galvanic cell is 3.09 volts when the work done to transfer the charge of 255 colombs is 788 joules.
The voltage of a galvanic cell can be calculated using the formula:
[tex]Voltage (V) = Work (J) / Charge (C)[/tex]
Given that the galvanic cell does 788 J of work and transfers 255 coulombs of charge, we can plug these values into the formula:
[tex]Voltage (V) = Work (J) / Charge (C)[/tex]
[tex]Voltage (V) = 788 J / 255 C = 3.09 V[/tex]
So, the voltage of the galvanic cell is approximately 3.09 volts.
Learn more about galvanic cell here:
https://brainly.com/question/13031093
#SPJ11
Base your answer on the information and illustrations below and on your knowledge of biology. The illustrations represent cross sections of two different plant stems.
A student compared two stem cross sections. Stem cross section A is from a plant that can be used to produce products with valuable medicinal properties. Stem cross section B is from a plant growing in the same area of the forest and its usefulness for producing medicines is unknown. The student concluded that the stem cross sections had many structural similarities and that the plant that produced cross section B would produce the same valuable medicinal products.
Is the student's conclusion valid?
A) Yes, because the structural similarities indicate a close relationship between the organisms.
B) Yes, because these plants grow in the same regions of the forest ecosystem and look similar.
C) No, because he did not evaluate soil conditions, such as pH, with chemical indicators.
D) No, because this structural evidence alone is insufficient and molecular evidence should be obtained.
Answers
Option D is the correct answer. This is because the production of medicinal compounds is determined by the plant's genetics and biochemistry, which may not be reflected in the plant's structural features alone.
What is the students conclusion?The student's conclusion is not valid. While the two stem cross sections may have many structural similarities, this is not sufficient evidence to conclude that the plant that produced cross section B will produce the same valuable medicinal products as the plant that produced cross section A.
Option A and B are incorrect because structural similarities do not necessarily indicate a close relationship between organisms or their biochemical properties. Option C is also incorrect because while soil conditions may affect plant growth, they do not necessarily determine a plant's ability to produce specific medicinal compounds.
Learn more about stem cross sections:https://brainly.com/question/1653214
#SPJ1
2. If 1.2 mol of tin metal and excess gold (III) chloride are used, how many moles of gold metal form? a. 0.67 mol
b. 0.80 mol
c. 1.2 mol
d. 2.0 mol
e. 2.4 mol
Answers
The number of moles for Gold metal when 1.2 mol of tin metal and excess gold (III) chloride are used is 0.8 mol, option B.
In chemistry, a mole, usually spelt mol, is a common scientific measurement unit for significant amounts of extremely small objects like atoms, molecules, or other predetermined particles.
The mole signifies 6.02214076 1023 units, which is a very big quantity. For the International System of Units (SI), the mole is defined as this quantity as of May 20, 2019, according the General Conference on Weights and Measures. The number of atoms discovered via experimentation to be present in 12 grammes of carbon-12 was originally used to define the mole.
In commemoration of the Italian physicist Amedeo Avogadro (1776–1856), the quantity of units in a mole is also known as Avogadro's number or Avogadro's constant. According to Avogadro, equivalent quantities of gases under same circumstances contain an identical number of molecules.
We have equation as,
2AuCl3 + 3Sn → 3SnCl2 + 2Au
If 3 moles of Tin metal gives us 2 moles of the Gold metal then,
1.2 moles of tin will give = 1.2 x 2 / 3 = 0.8 mol.
Therefore, number of mole for gold will be 0.8 mol.
Learn more about Number of moles:
https://brainly.com/question/13314627
#SPJ4
The number of moles for Gold metal when 1.2 mol of tin metal and excess gold (III) chloride are used is 0.8 mol, option B.
In chemistry, a mole, usually spelt mol, is a common scientific measurement unit for significant amounts of extremely small objects like atoms, molecules, or other predetermined particles.
The mole signifies 6.02214076 1023 units, which is a very big quantity. For the International System of Units (SI), the mole is defined as this quantity as of May 20, 2019, according the General Conference on Weights and Measures. The number of atoms discovered via experimentation to be present in 12 grammes of carbon-12 was originally used to define the mole.
In commemoration of the Italian physicist Amedeo Avogadro (1776–1856), the quantity of units in a mole is also known as Avogadro's number or Avogadro's constant. According to Avogadro, equivalent quantities of gases under same circumstances contain an identical number of molecules.
We have equation as,
2AuCl3 + 3Sn → 3SnCl2 + 2Au
If 3 moles of Tin metal gives us 2 moles of the Gold metal then,
1.2 moles of tin will give = 1.2 x 2 / 3 = 0.8 mol.
Therefore, number of mole for gold will be 0.8 mol.
Learn more about Number of moles:
brainly.com/question/13314627
#SPJ11
A buffer solution contains 0.24 M NH3 and 0.20 M NH4Cl.a. What is the pH of this buffer if Kb=1.8×10−5?b. What is the pH if 0.0050 moles of solid KOH is added to 0.500 L of this solution, assuming the total volume does not change?c. Briefly explain how the buffer capacity could be increased while maintaining the pH.
Answers
A buffer solution of [tex]NH[/tex]₃ and [tex]NH[/tex]₄[tex]Cl[/tex] with a pH of 9.25; adding [tex]KOH[/tex] increases pH to 9.54; buffer capacity can be increased by adding components.
a. To find the pH of this buffer, we can use the Henderson-Hasselbalch equation:
[tex]pH = pKa + log([A^-]/[HA])[/tex]
In this case, [tex]NH[/tex]₃ is the base (A⁻) and [tex]NH[/tex]₄⁺ is the conjugate acid (HA). The pKa can be calculated from the Kb:
[tex]Kw = Ka * Kb\\pKa + pKb = 14[/tex]
[tex]pKa = 14 - pKb = 14 - (-log10(1.8x10[/tex] ⁻ [tex]5)) = 9.54[/tex]
Substituting the values into the Henderson-Hasselbalch equation, we get:
pH = pKa + log([A⁻]/[HA])
= 9.54 + log(0.20/0.24)
= 9.25
Therefore, the pH of this buffer is 9.25.
b. When 0.0050 moles of solid [tex]KOH[/tex] is added to the buffer solution, it reacts with [tex]NH[/tex]₄⁺ (positively charged ammonium ion) to form [tex]NH[/tex]₃ and water:
[tex]KOH[/tex] + [tex]NH[/tex]₄⁺ → [tex]NH[/tex]₃ + [tex]H[/tex]₂[tex]O[/tex] + [tex]K[/tex]⁺
The number of moles of [tex]NH[/tex]₄⁺ initially present in the solution is:
0.20 M x 0.500 L = 0.100 moles
Since 0.0050 moles of [tex]KOH[/tex] are added, the remaining moles of [tex]NH[/tex]₄⁺ is:
0.100 - 0.0050 = 0.0950 moles
The number of moles of [tex]NH[/tex]₃ initially present in the solution is:
0.24 M x 0.500 L = 0.120 moles
Since [tex]NH[/tex]₄⁺ and [tex]NH[/tex]₃ react in a 1:1 stoichiometric ratio, the remaining moles of [tex]NH[/tex]₃ are also 0.0950 moles.
The total volume of the solution is still 0.500 L, so the new concentration of [tex]NH[/tex]₄⁺ is:
0.0950 moles / 0.500 L = 0.190 M
The new concentration of [tex]NH[/tex]₃ is also 0.190 M since the number of moles of [tex]NH[/tex]₃ and [tex]NH[/tex]₄⁺ are equal.
Using the Henderson-Hasselbalch equation again, we get:
[tex]pH = 9.54 + log([0.190]/[0.190])[/tex]
= 9.54
Therefore, the pH of the buffer after adding [tex]KOH[/tex] is 9.54.
c. The buffer capacity can be increased by adding more of the weak acid and its conjugate base to the solution. This increases the concentration of both the acid and its conjugate base, which in turn increases the buffer capacity. The pH can be maintained by adjusting the ratio of acid to base in the buffer. Another way to increase the buffer capacity is to increase the total volume of the buffer solution, which dilutes any added acid or base and reduces its effect on the pH.
Learn more about buffer solutions at
brainly.com/question/24262133
#SPJ4
How many molecules are present in 0. 340 g of HCl?
Answers
The molecular weight of HCl is approximately 36.46 g/mol (1.01 g/mol for hydrogen plus 35.45 g/mol for chlorine).
Using this information, we can calculate the number of moles of HCl in 0.340 g as follows:
moles of HCl = mass of HCl / molecular weight of HCl
moles of HCl = 0.340 g / 36.46 g/mol
moles of HCl = 0.00933 mol
Next, we can use Avogadro's constant, which is approximately 6.022 x 10^23 molecules/mol, to calculate the number of molecules of HCl in 0.340 g:
number of molecules of HCl = moles of HCl x Avogadro's constant
number of molecules of HCl = 0.00933 mol x 6.022 x 10^23 molecules/mol
number of molecules of HCl = 5.61 x 10^21 molecules
Therefore, there are approximately 5.61 x 10^21 molecules of HCl present in 0.340 g of HCl.
N (molecules) = 5.60 x 10^21 molecules
Explanation:
To calculate the number of molecules in a given mass of a substance, we need to use the following steps:
Calculate the number of moles of the substance using its molar mass.
Use Avogadro's number to convert the number of moles to the number of molecules.
The molar mass of HCl is the sum of the molar masses of hydrogen (1.008 g/mol) and chlorine (35.45 g/mol), which gives:
Molar mass of HCl = 1.008 g/mol + 35.45 g/mol = 36.458 g/mol
Using the given mass of HCl, we can now calculate the number of moles:
moles of HCl = mass of HCl / molar mass of HCl
moles of HCl = 0.340 g / 36.458 g/mol
moles of HCl = 0.00933 mol
Finally, we can convert the number of moles to the number of molecules using Avogadro's number:
number of molecules = moles of HCl x Avogadro's number
number of molecules = 0.00933 mol x 6.022 x 10^23 molecules/mol
number of molecules = 5.60 x 10^21 molecules
Therefore, there are approximately 5.60 x 10^21 molecules in 0.340 g of HCl.
what is the net cell reaction for the iron-silver voltaic cell? express your answer as a chemical equation.
Answers
The electrons already balance, so we can combine the reactions directly:
Fe (s) + 2Ag⁺ (aq) → Fe²⁺ (aq) + 2Ag (s)
The net cell reaction for the iron-silver voltaic cell involves two half-reactions. The anode half-reaction involves the oxidation of iron, while the cathode half-reaction involves the reduction of silver ions. The half-reactions can be expressed as follows:
Anode (oxidation): Fe (s) → Fe²⁺ (aq) + 2e⁻
Cathode (reduction): 2Ag⁺ (aq) + 2e⁻ → 2Ag (s)
To find the net cell reaction, we combine these half-reactions, ensuring that the number of electrons in the oxidation half-reaction equals the number of electrons in the half-reaction. In this case,
This is the net cell reaction for the iron-silver voltaic cell, represented as a chemical equation.
To learn more about : electrons
https://brainly.com/question/26084288
#SPJ11
More than 40 compounds in tobacco and tobacco smoke are. A) antioxidants. B) carcinogens. C) infectious agents. D) carcinomas.
Answers
More than 40 compounds in tobacco and tobacco smoke are option D: carcinogens.
The U.S. Food and Drug Administration created a list of dangerous and possibly toxic components in tobacco smoke and unburned tobacco in 2012; 79 of these substances are regarded as carcinogens. All tobacco products contain nicotine, a highly addictive substance that may be found in the tobacco plant itself.
While nicotine makes people addicted and keeps them using tobacco products, it is not the cause of the extreme danger associated with tobacco use. Numerous compounds are found in tobacco and tobacco smoke. It is this concoction of chemicals, not nicotine, that renders tobacco smokers susceptible to fatal illnesses.
To know more about carcinogens, refer:
https://brainly.com/question/24501446
#SPJ4
you need to prepare 250.0 ml of a 0.100 m aqueous solution using a pure solid with a molar mass of 278.5 g/mol. how many grams of solid should you use to make this solution?
Answers
we need to use 6.96 grams of the solid to prepare a 0.100 m aqueous solution with a volume of 250.0 ml.
To prepare a 0.100 m aqueous solution with a volume of 250.0 ml, we need to calculate the number of moles of the solute required using the formula:
Molarity = moles of solute / volume of solution in liters
0.100 mol/L = moles of solute / 0.250 L
moles of solute = 0.100 mol/L x 0.250 L = 0.025 mol
Now we can use the molar mass of the solid to calculate the mass required:
mass = moles of solute x molar mass
mass = 0.025 mol x 278.5 g/mol = 6.96 g
Therefore, we need to use 6.96 grams of the solid to prepare a 0.100 m aqueous solution with a volume of 250.0 ml.
To prepare a 250.0 mL of a 0.100 M aqueous solution using a pure solid with a molar mass of 278.5 g/mol, you will need to use the following formula:
mass (g) = volume (L) × molarity (M) × molar mass (g/mol)
First, convert the volume from mL to L:
250.0 mL = 0.250 L
Next, plug in the values into the formula:
mass (g) = 0.250 L × 0.100 M × 278.5 g/mol
Calculate the mass of the solid:
mass (g) = 6.9625 g
You should use 6.9625 grams of the solid to make the 250.0 mL of 0.100 M aqueous solution.
Visit here to learn more about molarity : https://brainly.com/question/8732513
#SPJ11
To prepare a 0.100 m aqueous solution with a volume of 250.0 ml, we need to use the formula:
moles of solute = Molarity x Volume (in liters)
First, we need to convert the volume from milliliters to liters:
250.0 ml = 0.250 L
Now, we can substitute the given values into the formula:
moles of solute = 0.100 mol/L x 0.250 L
moles of solute = 0.025 mol
Next, we need to calculate the mass of the solid we need to use. We can use the formula:
moles of solute = mass of solute / molar mass
Rearranging the formula, we get:
mass of solute = moles of solute x molar mass
Substituting the given values, we get:
mass of solute = 0.025 mol x 278.5 g/mol
mass of solute = 6.9625 g
Therefore, you should use 6.9625 grams of the solid to prepare a 250.0 ml of a 0.100 m aqueous solution.
To know more about mass of solute:
https://brainly.com/question/29482678
#SPJ11
the rates for the consumption or production of individual species are related to each other and to the overall reaction rate by the stoichiometric coefficients of the reaction:
Answers
A key idea in chemical kinetics is that "the rates for the consumption or production of individual species are related to each other and to the overall reaction rate by the stoichiometric coefficients of the reaction."
The rule of mass action, which states that the rate of a chemical reaction is proportional to the product of the reactant concentrations elevated to their stoichiometric coefficients, is the basis of this system.
To put it another way, if there is a chemical reaction:
aA + bB → cC + dD
where a, b, c, and d are the stoichiometric coefficients of the chemical species A, B, C, and D, the rate of this reaction can be written as follows:
rate = k [A] [A] a [B] [B]
where [A] and [B] are the concentrations of A and B, respectively, and k is the rate constant. The stoichiometry of the reaction is shown by the exponents a and b.
The amount of each species that is consumed or created during the reaction is likewise determined by the stoichiometric coefficients. In the reaction described above, for instance, if a = 2 and b = 1, then two molecules of A are wasted for each molecule of B that reacts.
Learn more about stoichiometric coefficients
https://brainly.com/question/29856106
#SPJ4
what is meant by the term lower in energy? which is lower in energy, a mixture of hydrogen and oxygen gases or liquid water? how do you know? which of the two is more stable? how do you know?
Answers
Liquid water is lower in energy and more stable than a mixture of hydrogen and oxygen gases because the bonds between its molecules are stronger and more difficult to break.
When we say something is "lower in energy," we mean it has less potential to do work or produce a reaction than something with higher energy. This is because energy is stored in the bonds between atoms and molecules, and the strength of those bonds determines the potential energy of the substance.
In the case of hydrogen and oxygen gases versus liquid water, liquid water is lower in energy. This is because the bonds between the hydrogen and oxygen atoms in water are stronger than the bonds between the hydrogen and oxygen molecules in the gas phase. When hydrogen and oxygen gas react to form water, energy is released as the stronger bonds are formed.
Liquid water is also more stable than a mixture of hydrogen and oxygen gases. Stability refers to a substance's ability to resist change or decay over time. Water is more stable because its strong bonds make it less likely to break apart or react with other substances, while the mixture of hydrogen and oxygen gases is highly reactive and can potentially explode or ignite.
For such more questions on Lower in energy:
https://brainly.com/question/29841618
#SPJ11
Carbon dioxide is reduced by using electrons obtained from inorganic molecules, such as ammonia or hydrogen gas by ______________ since they do not use solar energy.
Answers
Answer:
chemoautotrophs
Explanation:
A 25.0 mL sample of 0.400 M NH3(aq) is titrated with 0.400 M HCI(aq). What is the pH at the equivalence point? (Kb of NH3 = 1.8 x 10^-5) a. 2.72 b. 4.97 C. 7.00 d. 9.03 e. 11.28
Answers
At the equivalence point, moles of HCl equal moles of [tex]$NH_{3}$[/tex]. So, 0.01 moles of HCl is present in 25 mL, giving a pH of 7.00 (answer c).
The balanced chemical equation for the reaction between [tex]$NH_{3}$[/tex] and HCl is:
[tex]$NH_{3}$[/tex](aq) + HCl(aq) → NH₄Cl (aq)
At the equivalence point, all the [tex]$NH_{3}$[/tex] has reacted with the HCl, and the solution contains only NH₄Cl, which is the salt of a strong acid and weak base. The [NH₄]⁺ ion is acidic, and its hydrolysis produces. Therefore, the pH at the equivalence point can be calculated using the Kb value of [tex]$NH_{3}$[/tex] and the concentration of [NH₄]⁺+ ion in the solution.
The initial moles of [tex]$NH_{3}$[/tex] in the solution can be calculated as:
moles of [tex]$NH_{3}$[/tex]= volume of solution (L) × concentration of[tex]$NH_{3}$[/tex] (mol/L)
moles of [tex]$NH_{3}$[/tex] = 0.025 L × 0.400 mol/L
moles of [tex]$NH_{3}$[/tex] = 0.010 mol
Since [tex]$NH_{3}$[/tex] HCl reacts in a 1:1 ratio, the moles of HCl required to reach the equivalence point is also 0.010 mol.
Therefore, the volume of HCl required can be calculated as:
volume of HCl = moles of HCl / concentration of HCl
volume of HCl = 0.010 mol / 0.400 mol/L
volume of HCl = 0.025 L
At the equivalence point, the moles of [NH₄]⁺ ion produced is also 0.010 mol, and its concentration can be calculated as:
concentration of [NH₄]⁺ = moles of [NH₄]⁺ / volume of solution
concentration of [NH₄]⁺ = 0.010 mol / 0.050 L
concentration of [NH₄]⁺ = 0.200 mol/L
The Kb expression for [tex]$NH_{3}$[/tex] is:
Kb = [[tex]$NH_{3}$[/tex]][OH-] [NH₄]⁺
At the equivalence point, [[tex]$NH_{3}$[/tex]] = 0 and [NH₄]⁺ = 0.200 M. Therefore, the concentration of [tex]OH^-[/tex] can be calculated as:
Kb = [[tex]$NH_{3}$[/tex]][OH-] [NH₄]⁺
[tex]1.8 × 10^-5 = (0)([OH-]) / 0.200[/tex]
[OH-] = 0
Since [OH-] = 0, the concentration of [tex]H^+[/tex]at the equivalence point is equal to the concentration of [NH₄]⁺ ions, which is 0.200 M.
Therefore, the pH at the equivalence point can be calculated as:
pH = -log [tex]H^+[/tex]
pH = -log(0.200)
pH = 0.699
Therefore, the answer is (C) 7.00.
Learn more about equivalence point
https://brainly.com/question/11046523
#SPJ4
when you boil water, bubbles begin to form before the water boils. this happens because . question 12 options: the vapor pressure is increasing the water has salt dissolved in it it is simmering the dissolved air is coming out of the water
Answers
The dissolved air is coming out of the water, causing bubbles to form before the water boils. Option 4 is correct.
As the water is heated, the solubility of gases, such as air, decreases, causing the dissolved gases to be released as bubbles. This process is called nucleation and occurs at sites of imperfections in the container or impurities in the water, which provide a surface for the bubbles to form.
Once the water reaches its boiling point, the vapor pressure of the liquid equals atmospheric pressure, causing bubbles to form throughout the liquid, not just at the nucleation sites. Hence Option 4 is correct.
To learn more about vapor pressure, here
https://brainly.com/question/11864750
#SPJ4
What processes are necessary in order to turn sand into rock.
A. Compaction and cementation
B. Cooling and crystallization
C. Uplift and deposition
D. Weathering and erosion
Answers
Option A. The processes are necessary in order to turn sand into rock is Compaction and cementation
The cycles important to transform sand into rock are compaction and cementation. Compaction happens when layers of dregs are kept on top of one another, making the grains of sand become packed and diminishing the pore space between them. Cementation happens when minerals hasten out of water and fill in the leftover pore space, restricting the grains of sand together into a strong stone. This interaction is called lithification and it is the means by which most sedimentary rocks are shaped. Without compaction and cementation, sand would stay unconsolidated and not structure into a strong stone.
To learn more about Compaction and cementation, refer:
https://brainly.com/question/21543814
#SPJ4
most mp air masses that influence the u.s. originate over:
Answers
Most mP air masses that influence the U.S. originate over: the north Pacific.
The continent's air masses, which contain northern and southern components and are further separated into continental (dry) and marine (wet) types, reflect various temperature and humidity conditions. There are four types of air masses in the north: the Arctic air mass, which is over Greenland and the Canadian Arctic Archipelago; the polar continental; the maritime polar Pacific; and the maritime polar Atlantic, which is off the Atlantic coasts of Canada and New England.
The subtropical maritime Pacific air mass, located off the southwestern United States, the tropical continental air mass, located over the intermontane Cordillera basins from Utah southward, and the maritime tropical air mass, centred over the Gulf of Mexico and the Caribbean, are what define the continent's southern half.
Learn more about Air masses:
https://brainly.com/question/26209372
#SPJ4
Most maritime (mP) polar air masses that influence the U.S. originate over the North Pacific and North Atlantic Oceans
Most maritime polar (mP) air masses that influence the United States originate over the North Pacific and the North Atlantic oceans. These air masses are characterized by their cool and moist nature, as they form over relatively colder ocean waters. They often bring cloudy and wet weather to the regions they affect, especially along the Pacific Northwest coast and the northeastern seaboard of the United States. Most maritime polar (mP) air masses that influence the U.S. originate over the North Pacific and North Atlantic Oceans. These air masses bring cool, moist conditions to coastal regions of the country.
To learn more about polar air masses click here
brainly.com/question/15301156
#SPJ11
for how many minutes must a current of 1.4 amp be provided to deliver 890 coulombs?group of answer choices121191010.595
Answers
A current of 1.4 amp must be provided for approximately 635.71 seconds, or about 10.59 minutes, to deliver 890 coulombs of charge.
The time required to deliver a certain amount of charge is directly proportional to the amount of charge and inversely proportional to the current.
We can use the formula:
charge (Q) = current (I) x time (t)
to solve for the time required. Rearranging the formula gives:time (t) = charge (Q) / current (I)
Substituting the given values, we get:
time (t) = 890 coulombs / 1.4 amp = 635.71 seconds
Therefore, a current of 1.4 amp must be provided for approximately 635.71 seconds, or about 10.59 minutes, to deliver 890 coulombs of charge.
Learn more about coulombs ,
https://brainly.com/question/12498766
#SPJ4
A current of 1.4 amps must be provided for 635 seconds (or approximately 10.6 minutes) to deliver 890 coulombs.
To deliver 890 coulombs with a current of 1.4 amps, we can use the formula:
Q = I x t
where Q is the charge in coulombs, I is the current in amperes, and t is the time in seconds.
We need to find t, so we can rearrange the formula to solve for t:
t = Q / I
Plugging in the values we have:
t = 890 coulombs / 1.4 amps
t = 635 seconds
To find the time (in minutes) needed to deliver 890 Coulombs with a current of 1.4 Amps, use the formula Q = I*t, where Q is the charge in Coulombs, I is the current in Amps, and t is the time in seconds.
1. First, solve for t: t = Q/I
2. Plug in the values: t = 890/1.4
3. Calculate t: t ≈ 635.71 seconds
To convert seconds to minutes, divide by 60:
4. t ≈ 635.71/60
5. t ≈ 10.595 minutes
So, a current of 1.4 Amps must be provided for approximately 10.595 minutes to deliver 890 Coulombs.
To learn more about amps click here
brainly.com/question/4692514
#SPJ11
the ph of a 0.115m solution of chloroacetic acid, clch2cooh, is measured to be 1.92. calculate ka for this monoprotic acid.
Answers
The Ka value for chloroacetic acid is 1.4 x 10^-3.
The pH of a 0.115M solution of chloroacetic acid (ClCH2COOH) was measured to be 1.92. To determine the acid dissociation constant (Ka) for this monoprotic acid,
we can use the formula Ka = [H3O+][ClCH2COO-]/[ClCH2COOH]. To begin, we first need to find the concentration of H3O+ ions in solution. Since pH is defined as -log[H3O+],
we can rearrange the formula to find [H3O+] = 10^-pH. Substituting the pH value of 1.92 into this equation gives us [H3O+] = 6.31 x 10^-2 M. We can then use the equation for Ka and substitute the appropriate values to obtain Ka = (6.31 x 10^-2)^2 / (0.115 - 6.31 x 10^-2) = 1.4 x 10^-3.
To learn more about : chloroacetic
https://brainly.com/question/17137710
#SPJ11
aqueous hydrobromic acid will react with solid sodium hydroxide to produce aqueous sodium bromide and liquid water . suppose 4.05 g of hydrobromic acid is mixed with 3.7 g of sodium hydroxide. calculate the maximum mass of sodium bromide that could be produced by the chemical reaction. be sure your answer has the correct number of significant digits.
Answers
, the maximum mass of NaBr that can be produced is 5.14 g (rounded to two significant figures to match the significant figures in the given masses of reactants).
balanced chemical equation for the reaction between hydrobromic acid and sodium hydroxide is:
HBr (aq) + NaOH (s) → NaBr (aq) + H₂O (l)
To determine the maximum mass of sodium bromide that can be produced, we need to first calculate the limiting reactant, which is the reactant that is completely consumed in the reaction.
The molar mass of HBr is 80.91 g/mol, and the molar mass of NaOH is 40.00 g/mol. Using these values, we can calculate the number of moles of each reactant:
moles of HBr = 4.05 g / 80.91 g/mol = 0.050 mol
moles of NaOH = 3.7 g / 40.00 g/mol = 0.0925 mol
Since NaOH has a higher number of moles, it is in excess, and HBr is the limiting reactant.
Using the balanced chemical equation, we can now calculate the theoretical yield of NaBr:
1 mol HBr produces 1 mol NaBr
0.050 mol HBr produces 0.050 mol NaBr
The molar mass of NaBr is 102.89 g/mol, so the mass of NaBr produced is:
mass of NaBr = 0.050 mol × 102.89 g/mol = 5.1445 g
Learn more about reactants here:
https://brainly.com/question/17096236
#SPJ11
The maximum mass of sodium bromide that could be produced by this reaction is approximately 5.14 g, considering the correct number of significant digits.
How to determine the yield of a reaction?To calculate the maximum mass of sodium bromide that could be produced by the reaction of aqueous hydrobromic acid and solid sodium hydroxide, we'll follow these steps:
1. Write the balanced chemical equation: HBr(aq) + NaOH(s) → NaBr(aq) + H₂O(l)
2. Calculate the moles of reactants:
- For HBr (molecular weight = 80.91 g/mol): moles = 4.05 g / 80.91 g/mol ≈ 0.0500 mol
- For NaOH (molecular weight = 40.00 g/mol): moles = 3.7 g / 40.00 g/mol ≈ 0.0925 mol
3. Determine the limiting reactant: Since the stoichiometry is 1:1, HBr is the limiting reactant with 0.0500 mol.
4. Calculate the moles of NaBr produced: 0.0500 mol HBr × (1 mol NaBr / 1 mol HBr) = 0.0500 mol NaBr
5. Calculate the mass of NaBr produced (molecular weight = 102.89 g/mol): mass = 0.0500 mol × 102.89 g/mol ≈ 5.14 g
To know more about Yield:
https://brainly.com/question/29655076
#SPJ11
which acid in table 14.2 is most appropriate for preparation of a buffer solution with a ph of 3.7? explain your choice.
Answers
We can create a buffer solution with a pH of 3.7 by using formic acid as the buffer system's acid component.
What pH does a buffer solution have?To keep fundamental conditions in place, these buffer solutions are used. A weak base and its salt are combined with a strong acid to create a basic buffer, which has a basic pH. Aqueous solutions of ammonium hydroxide and ammonium chloride at equal concentrations have a pH of 9.25. These solutions have a pH greater than seven.
Why may the pH of a buffered solution resist changing?When little amounts of acid or base are supplied, buffers can resist pH changes, because they have an acidic component (HA) to neutralise OH- ions and a basic component (A-) to neutralise H+ ions, they are able to accomplish this.
To know more about buffer solution visit:-
https://brainly.com/question/24262133
#SPJ1
for the dyes synthesized from a naphthol starting material, did the position of the hydroxyl group an effect on the wavelength of light that was absorbed by the dyes? explain g
Answers
Yes, the position of the hydroxyl group does have an effect on the wavelength of light absorbed by the dyes synthesized from a naphthol starting material.
This is because the position of the hydroxyl group determines the electronic properties of the molecule, which in turn affects the energy levels and transitions that occur when the molecule absorbs light. In general, molecules with hydroxyl groups attached to positions closer to the aromatic ring will absorb light at shorter wavelengths (higher energy), while those with hydroxyl groups attached to positions farther from the ring will absorb light at longer wavelengths (lower energy).
This phenomenon is known as the bathochromic or hypsochromic effect, depending on whether the shift is toward longer or shorter wavelengths, respectively.
To learn more about bathochromic or hypsochromic effect, here
https://brainly.com/question/14083655
#SPJ4
how many molecules of lithium chloride are in 78.40 g? the molar mass of lithium chloride is 42.39 gmol.
Answers
Answer:
To find the number of molecules of lithium chloride in 78.40 g, we need to first convert the mass to moles using the molar mass of lithium chloride:
moles = mass / molar mass
moles = 78.40 g / 42.39 g/mol
moles = 1.849 mol
Now we can use Avogadro's number to convert from moles to molecules:
molecules = moles x Avogadro's number
molecules = 1.849 mol x 6.022 x 10^23 molecules/mol
molecules = 1.111 x 10^24 molecules
Therefore, there are 1.111 x 10^24 molecules of lithium chloride in 78.40 g.
Explanation:
In order to prepare 2.00 L of a 3.00 M solution of ferric chloride (FeCl3) how many grams of ferric chloride must be used
Answers
We need to use 973.24 grams of ferric chloride to prepare 2.00 L of a 3.00 M solution of FeCl₃.
Describe Mass.Mass is a fundamental physical quantity that represents the amount of matter in an object. It is a scalar quantity and is measured in units of kilograms (kg) or grams (g). Mass is not the same as weight, which is the force exerted on an object due to gravity and varies with the strength of the gravitational field.
The mass of an object is determined by its inertia, which is the resistance to acceleration that an object exhibits due to its mass. The greater the mass of an object, the greater its inertia and the more force is required to accelerate it. Mass is a conserved quantity, meaning that it cannot be created or destroyed, only transferred or transformed through physical or chemical processes.
To calculate the mass of ferric chloride needed to prepare a 3.00 M solution of FeCl₃, we need to use the formula:
Molarity (M) = moles of solute / volume of solution (in liters)
Rearranging this formula gives:
moles of solute = Molarity x volume of solution
We can then use the molar mass of FeCl₃ to convert moles of solute to grams of FeCl₃. The molar mass of FeCl₃ is:
FeCl₃ = 55.845 + 3(35.453) = 162.206 g/mol
So, to prepare 2.00 L of a 3.00 M solution of FeCl₃, we have:
moles of FeCl₃ = Molarity x volume of solution
moles of FeCl₃ = 3.00 mol/L x 2.00 L
moles of FeCl₃ = 6.00 mol
mass of FeCl₃ = moles of FeCl3 x molar mass of FeCl3
mass of FeCl₃ = 6.00 mol x 162.206 g/mol
mass of FeCl₃ = 973.24 g
Therefore, we need to use 973.24 grams of ferric chloride to prepare 2.00 L of a 3.00 M solution of FeCl₃V.
To know more about moles, visit:
https://brainly.com/question/30308829
#SPJ1
At what temperature will a sample of neon gas exert a pressure of 0. 750 atm and exhibit a density of 1. 45 gL
Answers
The sample of neon gas will exert a pressure of 0.750 atm and exhibit a density of 1.45 g/L at a temperature of 61.8 K.
To solve this problem, we can use the Ideal Gas Law, which relates the pressure, volume, number of moles, and temperature of a gas:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
We can rearrange this equation to solve for T:
T = PV / (nR)
We are given the pressure (P = 0.750 atm), the density (ρ = 1.45 g/L), and the molecular weight of neon (20.18 g/mol).
First, we need to calculate the number of moles of neon:
n = m / M
where m is the mass of the sample and M is the molecular weight.
Since we are not given the mass, we can use the density to calculate it:
ρ = m / V
m = ρV = 1.45 g/L × V
Now we can substitute this expression for m into the equation for n:
n = (1.45 g/L × V) / 20.18 g/mol
Next, we need to calculate the volume of the gas at the given conditions. We can use the density to do this:
ρ = nM / V
V = nM / ρ = (n × 20.18 g/mol) / 1.45 g/L
Now we can substitute the expressions for n and V into the equation for T:
T = PV / (nR) = (0.750 atm) × [(n × 20.18 g/mol) / 1.45 g/L] × (0.0821 L atm/mol K)⁻¹
Simplifying this expression gives:
T = 61.8 K
To know more about pressure, here
brainly.com/question/29567868
#SPJ4
A 5. 0 L sample of gas is collected at 400. MmHg at 727 C. What is the volume if the temperature were cooled to 77 C and the pressure increased to 700. MmHg?
Answers
The volume would be approximately 0.71 L if the temperature were cooled to 77 °C and the pressure increased to 700 mmHg.
We will use the combined gas law to solve this problem;
P₁V₁/T₁ = P₂V₂/T₂
where P₁, V₁, as well as T₁ are the initial pressure, volume, and the temperature, respectively, and P₂, V₂, and T₂ will be the final pressure, volume, as well as temperature, respectively.
Plugging in the given values, we get;
(400 mmHg)(5.0 L)/(1000 K) = (700 mmHg)(V₂)/(350 K)
Simplifying and solving for V₂, we get;
V₂ = (400 mmHg)(5.0 L)(350 K)/(700 mmHg)(1000 K)
V₂ ≈ 0.71 L
To know more about combined gas law here
https://brainly.com/question/13154969
#SPJ4
determine the standard enthalpy change for the decomposition of hydrogen peroxide per mole of hydrogen peroxide.
Answers
The standard enthalpy change for the decomposition of hydrogen peroxide per mole of hydrogen peroxide is -98.2 kJ/mol.
when 1 mole of hydrogen peroxide (H2O2) ( H 2 O 2 ) undergoes decomposition, the heat evolved (ΔH) is −98.2kJ. − 98.2 k J . The molar mass of H2O2 H 2 O 2 is 34.015 g/mol. This means that the mass of 1 mole of H2O2 H 2 O 2 is 34.015 g.
This value is obtained from the standard enthalpy of formation of the products (H2 and O2) and the standard enthalpy of formation of the reactant (H2O2). Enthalpy of formation is the energy change that occurs when a compound is formed from its elements, in their standard states.
The difference between the enthalpies of formation of the products and the reactant is the enthalpy change for the reaction. In this case, the enthalpy change for the decomposition of hydrogen peroxide is -98.2 kJ/mol. This indicates that the decomposition of hydrogen peroxide is an exothermic reaction and it releases 98.2 kJ/mole of energy.
Know more about Hydrogen peroxide here
https://brainly.com/question/29102186#
#SPJ11
which observation best describes the physical appearance of a compound when the end of its melting point range is reached? the compound begins to convert to a liquid. the compound completely converts to a liquid. the compound begins to evaporate.
Answers
A compound turns completely into a liquid this observation best describes the physical appearance of a compound when it reaches the end of its melting point range. Here option B is the correct answer.
When a solid compound is heated, it undergoes a process called melting in which it transforms into a liquid state. The melting point of a compound is the temperature at which it changes from a solid to a liquid state. The melting process is characterized by a range of temperatures over which the compound is observed to be partially or fully melted.
The observation that best describes the physical appearance of a compound when the end of its melting point range is reached is B - the compound completely converts to a liquid. At the end of the melting point range, the compound has absorbed enough heat energy to fully overcome the intermolecular forces that hold its constituent particles together in a solid state, resulting in the complete transformation of the compound into a liquid.
This state is characterized by the loss of a crystalline structure, where the particles are free to move about and slide past each other, leading to an increased fluidity and mobility of the compound. At this stage, the compound is fully melted and can be poured or transferred into a new container in its liquid form.
To learn more about melting points
https://brainly.com/question/28902417
#SPJ4
Complete question:
Which observation best describes the physical appearance of a compound when the end of its melting point range is reached?
A - the compound begins to convert to a liquid.
B - the compound completely converts to a liquid.
C - the compound begins to evaporate.