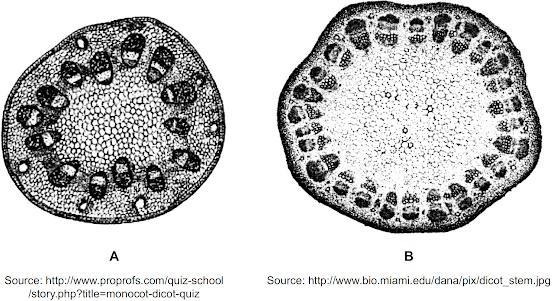
Base your answer on the information and illustrations below and on your knowledge of biology. The illustrations represent cross sections of two different plant stems.
A student compared two stem cross sections. Stem cross section A is from a plant that can be used to produce products with valuable medicinal properties. Stem cross section B is from a plant growing in the same area of the forest and its usefulness for producing medicines is unknown. The student concluded that the stem cross sections had many structural similarities and that the plant that produced cross section B would produce the same valuable medicinal products.
Is the student's conclusion valid?
A) Yes, because the structural similarities indicate a close relationship between the organisms.
B) Yes, because these plants grow in the same regions of the forest ecosystem and look similar.
C) No, because he did not evaluate soil conditions, such as pH, with chemical indicators.
D) No, because this structural evidence alone is insufficient and molecular evidence should be obtained.
Answers
Option D is the correct answer. This is because the production of medicinal compounds is determined by the plant's genetics and biochemistry, which may not be reflected in the plant's structural features alone.
What is the students conclusion?The student's conclusion is not valid. While the two stem cross sections may have many structural similarities, this is not sufficient evidence to conclude that the plant that produced cross section B will produce the same valuable medicinal products as the plant that produced cross section A.
Option A and B are incorrect because structural similarities do not necessarily indicate a close relationship between organisms or their biochemical properties. Option C is also incorrect because while soil conditions may affect plant growth, they do not necessarily determine a plant's ability to produce specific medicinal compounds.
Learn more about stem cross sections:https://brainly.com/question/1653214
#SPJ1
Related Questions
how many electrons are removed from c6h12o6 during cellular respiration when its broken down into 6co2 and water
Answers
The total of 12 electrons are removed from glucose during cellular respiration to produce 6CO2 and water.
During cellular respiration, glucose (C6H12O6) is broken down into carbon dioxide (6CO2) and water. This process involves the removal of electrons from glucose molecules, which are then used to create ATP, the energy currency of cells.
Specifically, in glycolysis, two electrons are removed from glucose to form NADH, which carries these electrons to the electron transport chain (ETC) for further energy production. In the ETC, the electrons are transferred between different electron carriers and ultimately used to produce ATP.
It is important to note that cellular respiration is a complex process that involves multiple steps and different electron carriers, and the removal of electrons from glucose is not a single event but a continuous process that generates energy for cells.
To learn more about : glucose
https://brainly.com/question/30174368
#SPJ11
if the size of the zinc electrode were doubled, does the cell voltage increase, decrease or stay the same? justify your answer.
Answers
In changing the size of the zinc electrode would not impact the voltage of the cell.
If the size of the zinc electrode were doubled, the cell voltage would stay the same. This is because the voltage of a cell is dependent on the difference in potential between the two electrodes, not their size.
Doubling the size of the zinc electrode would not change the potential difference between the zinc and copper electrodes, therefore the cell voltage would remain constant.
The only factor that would affect the cell voltage would be a change in the concentration or temperature of the electrolyte solution or a change in the material or surface area of the copper electrode.
To learn more about : electrode
https://brainly.com/question/18251415
#SPJ11
Arrange the following events in the proper order in which they occur during an allergic response.
1 = Individual experiences symptoms
2 = Individual is sensitized to antigen
3 = IgE attaches to mast cells
4 = Antigen binds to IgE
Answers
Answer:
In order is 2, 3, 4, 1
Explanation:
It starts when you come into contact with a trigger that you inhale, swallow, or get on your skin. In response, your body starts to make a protein called IgE, which grabs onto the allergen. Then histamine and other chemicals get released into the blood.
Which substance is not a structural isomer of hexyne?
a) hex-2-yne
b) hex-3-yne
c) 3,3-dimethylpent-1-yne
d) 4-methylpent-1-yne
e) 2,3-dimethylbuta-1,3-diene
Answers
2,3-dimethylbuta-1,3-diene is not a structural isomer of hexyne. Option e is correct.
Structural isomers are molecules with the same chemical formula but different arrangements of atoms. Hexyne is a hydrocarbon with six carbon atoms and one triple bond. Option (e), 2,3-dimethylbuta-1,3-diene, is not a structural isomer of hexyne because it has a different number of carbon atoms and a different type of bond. It has four carbon atoms and two double bonds, whereas hexyne has six carbon atoms and one triple bond.
Options (a), (b), (c), and (d) are all structural isomers of hexyne because they have the same number of carbon atoms and the same type of bond but different arrangements of atoms. Hence, option e is correct.
To know more about isomer, here
brainly.com/question/13422357
#SPJ4
Boyle's Law: If it takes 0.0500L of oxygen gas kept in a cylinder under pressure to fill an evacuated 4.00L reaction vessel in which the pressure is 0.980atm. What was the initial pressure of the gas in the cylinder?
Answers
P1V1 = P2V2
where P1 and V1 are the pressure and volume at the initial state, and P2 and V2 are the pressure and volume at the final state.
We are given:
V1 = 0.0500 L
V2 = 4.00 L
P2 = 0.980 atm
We can solve for P1:
P1 = (P2V2) / V1
P1 = (0.980 atm x 4.00 L) / 0.0500 L
P1 = 78.4 atm (rounded to one decimal place)
Therefore, the initial pressure of the gas in the cylinder was about 78.4 atm.
what mass of cu(s) is electroplated by running 23.0 a of current through a cu2 (aq) solution for 4.00 h ? express your answer to three significant figures and include the appropriate units.
Answers
Electrolysis is a process that is used to electric current is passed in a solution. The mass of cu(s) is electroplated by running 23.0 a of current through a cu2 (aq) solution for 4.00 h is equals to 64 grams.
Electrolysis is a process in which an electric current is passed in a solution. Solving electrolysis problem is more on stoichiometric calculations are, coulombs = amperes x time
1 Faraday = 96,485 coulombs
1 Faraday = 1 mole of electrons
We have to determine the mass of cu(s) is electroplated by running 23.0 a of current through a Cu (aq) solution for 4.00 h. Half reaction, [tex]Cu^{2+ } + 2e^{-} --> Cu[/tex]
Current, I = 23.0 A
Time, t = 4 hours = 4 × 3600 seconds
= 14400 seconds
Calculate the moles of Copper, n=Q ×z× F
where, Q = total charge in coulombs
F = Faraday constant = 96485 per molez = the number of electrons in the half-cell reaction = 2Computing for Q = 13.5coulomb sec (14,400 sec) = 194,400 coulomb-sec²
So, n = 194,400 coulomb-s² /(96485 coulomb)
= 1.007 moles Cu
Molar mass = 63.55 grams per mole
Molar mass is defined as the mass of substance divided by moles of substance.
=> 63.55 grams per mole = m/ 1.007 moles Cu
=> m = 63.55 g × 1.007
=> m = 64 grams
Hence, required value is 64 grams.
For more information about electrolysis, refer:
https://brainly.com/question/24063038
#SPJ4
if you are performing the following reaction using 81.3 g of the starting alcohol and 48.5 g of hydrobromic acid, what is your limiting reagent?
Answers
We can see that the hydrobromic acid is the limiting reagent because it is completely consumed when 0.599 mol of it reacts with 0.599 mol of ethanol. After the reaction is complete, there will be some excess ethanol left over.
To determine the limiting reagent, we need to compare the amount of moles of each reactant used in the reaction. We can calculate the number of moles of each reactant by dividing their mass by their molar mass. Let's assume the starting alcohol is ethanol and has a molar mass of 46.07 g/mol, and hydrobromic acid has a molar mass of 80.91 g/mol. Then we have:
Moles of ethanol = 81.3 g / 46.07 g/mol = 1.765 mol
Moles of hydrobromic acid = 48.5 g / 80.91 g/mol = 0.599 mol
According to the balanced chemical equation, the stoichiometric ratio of ethanol to hydrobromic acid is 1:1.
For such more questions on Hydrobromic acid:
https://brainly.com/question/31367861
#SPJ11
g standard conditions include a concentration of 1.0 m for soluble aqueous species, pure solids and liquids, and a partial pressure of 1 atm for gaseous species. group of answer choices true false previousnext
Answers
True. The standard conditions for measuring thermodynamic properties such as enthalpy, entropy, and Gibbs free energy are well-defined and standardized.
These conditions are used to compare and evaluate the relative stability and reactivity of different chemical species. The standard conditions for measuring these properties include a concentration of 1.0 m for soluble aqueous species, pure solids and liquids, and
a partial pressure of 1 atm for gaseous species. This means that the molar concentration of soluble aqueous species is set at 1.0 mol/L, and the pressure of gaseous species is set at 1 atm.
Pure solids and liquids are considered to have an activity of 1, which means that they do not affect the thermodynamic properties.
These conditions are used to determine the standard thermodynamic properties of chemical reactions, which are used to predict the direction and extent of chemical reactions.
To learn more about : standard
https://brainly.com/question/29136034
#SPJ11
A team of botanists conducted an experiment
investigating the effect of pH on plant growth.
The height of the plant was measured three weeks
after planting.
1
?
3.
Based on the data they collected, what is the
optimal pH for growing basil? Explain your
answer.
Based on the data they collected, which
plant fares better than the others in low pH
environments? Explain your answer.
At which pH is there the greatest difference
between the heights of parsley and basil?
What is the height difference at that pH?
Answers
The outcomes to the scan had been now not all similar. The pots with the pH of 5.0 had no growth whatsoever. The pots with the pH of 6.0 had little growth, each with only four blades of grass. The pots with a pH of 7.0 grew well, one pot with extra blades of grass than the other, an average of 11 blades of grass
What are the elements that affect the pH of a plant environment?Natural soil pH depends on the rock from which the soil was once fashioned (parent material) and the weathering procedures that acted on it—for instance climate, vegetation, topography and time. These approaches have a tendency to purpose a decreasing of pH (increase in acidity) over time.
There is disruption of nutrient absorption by way of the plants if it's pH increases, and hence, soil fertility is reduced, alkaline soil's pH does not lead to make bigger in nutrient absorption, soil illness does not happen.
Learn more about effect of pH on plant growth here:
https://brainly.com/question/31459436#SPJ1consider the pictured structure of a dipeptide. dipeptide structure with labels a through d. the a label is at the end of the molecule with the positively charged nh3 group. the b label is placed by the bond between the carbonyl group and the amine. the c label is next to a carbon with shown single bonds to the carboxylate, a ch2oh, and the rest of the molecule. the d label is next to the carboxylate at the end of the molecule. what does each label on the structure represent?
Answers
The dipeptide structure with labels a through d represents the different functional groups and atoms present in the molecule.
Label a is located at the end of the molecule with the positively charged NH3 group, indicating the presence of an amino group. The b label is placed by the bond between the carbonyl group and the amine, indicating the presence of a peptide bond.
Label c is next to a carbon with shown single bonds to the carboxylate, a CH2OH, and the rest of the molecule, indicating the presence of a side chain. Finally, the d label is next to the carboxylate at the end of the molecule,
indicating the presence of a carboxylic acid functional group. Understanding the different functional groups and atoms present in the dipeptide structure is important in understanding its properties and behavior in chemical reactions and biological processes.
To learn more about : labels
https://brainly.com/question/29608807
#SPJ11
Explain how Avogadro’s number can give two conversion factors
Answers
Answer: NA = no of molecules / no of moles
NA = no of molecules × molecular weight /weight
Explanation:
Question 9 (2 points) (10.03 MC) In a few sentences, describe what this weather map tells you about the weather. (2 points) L H
Answers
This weather map shows that there is a low pressure system in the north and a high pressure system in the south.
What is weather?Weather is the study of atmospheric conditions that exist in a specific area over a short period of time. It is the sum of all atmospheric conditions including temperature, humidity, wind, air pressure, cloud cover and precipitation. Weather is an important factor in determining the temperature, humidity and other characteristics of the environment. It affects human activities such as agriculture, transportation and recreation. Weather is dynamic and constantly changing. It is affected by a variety of factors such as solar radiation, air pressure, ocean currents, land topography and human activities. Weather is also affected by climate, which is the average weather pattern over a long period of time. Understanding weather is important for many reasons, including to predict storms and floods, to plan for extreme weather events, and to prepare for natural disasters.
This weather map shows that there is a low pressure system in the north and a high pressure system in the south. The low pressure system is bringing cooler temperatures and precipitation, while the high pressure system is bringing warmer temperatures and clear skies. There is a cold front moving eastward from the north, and a warm front moving eastward from the south.
To learn more about weather
https://brainly.com/question/29709289
#SPJ9
consider the reaction performed in the sn1 lab. what would be the effect on the rate of the reaction if 2-propanol (isopropanol) was used instead of 2-methyl-2-propanol (t-butanol) assuming only an sn1 reaction occurs? group of answer choices the rate of the reaction would decrease, because the secondary carbocation is more difficult to form. the rate of the reaction would increase, because the secondary carbocation is easier to form. there would be no difference in reaction rate. the reaction would not proceed at all.
Answers
The rate of the reaction is directly proportional to the stability of the carbocation intermediate, and any changes in the solvent will affect the rate of the reaction.
In an SN1 reaction, the rate-determining step is the formation of a carbocation intermediate. The stability of the carbocation intermediate affects the rate of the reaction.
In this case, if 2-propanol (isopropanol) was used instead of 2-methyl-2-propanol (t-butanol), the rate of the reaction would decrease. This is because the carbocation intermediate formed in 2-propanol is less stable compared to the one formed in t-butanol.
The carbocation intermediate formed in t-butanol is tertiary, which is more stable than the one formed in isopropanol, which is secondary. This means that the reaction will be slower in isopropanol due to the less stable carbocation intermediate.
To learn more about : reaction
https://brainly.com/question/29470602
#SPJ11
The cloud droplets in a cloud are formed by water vapor molecules and: A) protons. B) ions. C) molecules of air. D) condensation nuclei.
Answers
Answer:
condensation nuclei
Explanation:
can you guys help me with this question
Answers
Answer: flowing water
Explanation:
a sample of br2(g) takes 14.0 min to effuse through a membrane. how long would it take the same number of moles of ar(g) to effuse through the same membrane?
Answers
A sample of [tex]Br_2[/tex](g) takes 14.0 min to diffuse out through a membrane. It would take 7 min for the same number of moles of Ar(g) to effuse through the same membrane.
According to Graham's Law of Diffusion, it is known that the rate of diffusion of a gas is proportional to the reciprocal of the square root of the molar mass of the gas. The rate of diffusion is recorded under the same pressure and temperature conditions.
It can be written as [tex]\frac{r_1}{r_2}[/tex] ∝ [tex]\sqrt\frac{m_2}{m_1}[/tex]
where [tex]r_1[/tex] is the rate of diffusion of one of the gas
[tex]r_2[/tex] is the rate of diffusion of the second gas
[tex]r_1[/tex] is the molar mass of one of the gas
[tex]m_2[/tex] is the molar mass of the second gas
According to the question,
[tex]\frac{14}{r_2}=\sqrt\frac{160}{40}\\\frac{14}{r_2}=\sqrt\frac{4}{1} \\\\r_2 = 14\sqrt{\frac{1}{4} } = 7[/tex]
Therefore the time taken for the diffusion of Ar (g) is 7 min
Learn more about Diffusion:
https://brainly.com/question/29064792
#SPJ4
It would take approximately 7.0 minutes for the same number of moles of Ar(g) to effuse through the same membrane.
Using Graham's law of effusion, we can compare the rates of effusion for Br2(g) and Ar(g). The formula for Graham's law is:
Rate₁ / Rate₂ = √(M₂ / M₁)
Here, Rate₁ and Rate₂ are the effusion rates of the two gases, and M₁ and M₂ are their molar masses. In this case, Br2(g) is gas 1 and Ar(g) is gas 2. The molar mass of Br2 is 159.8 g/mol, and the molar mass of Ar is 39.95 g/mol.
Since we know the time it takes for Br2 to effuse, we can write:
Time₁ / Time₂ = Rate₂ / Rate₁ = √(M₁ / M₂)
Plugging in the given time and molar masses:
14.0 min / Time₂ = √(159.8 g/mol / 39.95 g/mol)
Solving for Time₂:
Time₂ = 14.0 min * √(39.95 g/mol / 159.8 g/mol) ≈ 7.0 min
Learn more about Graham's law of effusion here: brainly.com/question/22359712
#SPJ11
What is the concentration (in molality) of an aqueous solution of NaCl made by adding
4.56 g of NaCl to enough water to give 20.0 mL of solution. Assume the density of the
solution is 1.03 g/mL
Answers
Answer:
data given
mass of NaCl 4.56
dissolved volume 20ml(0.02l)
density of solution 1.03g/ml
Required molality
Explanation:
molarity=m/mr×v
where
m is mass
mr molar mass
v is volume
now,
molarity=4.56/58.5×0.02
molarity =3.9
: .molarity is 3.9mol/dm^3
According to molal concentration, the concentration (in molality) of an aqueous solution of NaCl is 0.0047 mole/kg.
What is molal concentration?Molal concentration is defined as a measure by which concentration of chemical substances present in a solution are determined. It is defined in particular reference to solute concentration in a solution . Most commonly used unit for molal concentration is moles/kg.
The molal concentration depends on change in volume of the solution which is mainly due to thermal expansion. Molal concentration is calculated by the formula, molal concentration=mass/ molar mass ×1/mass of solvent in kg.
In terms of moles, it's formula is given as molal concentration= number of moles /mass of solvent in kg.
Substitution in formula gives the answer but first mass of solution is determined which is density×volume= 1.03×20=20.6 g , mass of solvent= 20.6-4.56=16.05, thus molal concentration=4.56/58.5×1/16.05=0.0047 moles/kg.
Learn more about molal concentration,here:
https://brainly.com/question/4580605
#SPJ2
Each student will write up their own lab report and turn it in
Answers
Here are some general steps you can follow to write a lab report:
The StepsUnderstand the purpose of the lab report: Before you begin writing, make sure you understand the purpose of the lab report. What are the objectives of the experiment? What are the research questions being investigated? What hypothesis is being tested?
Gather your data: Make sure you have all the data you need to write your report. This includes raw data, observations, and any notes you took during the experiment. Organize your data in a clear and logical manner so that you can easily refer to it when writing your report.
Write an outline: Create an outline for your report that includes the main sections you need to cover. These typically include an introduction, methods, results, discussion, and conclusion.
Write the introduction: The introduction should provide an overview of the experiment and explain its significance. You should also provide some background information to help the reader understand the context of the experiment.
Write the methods: In the methods section, describe the experimental design, materials used, and procedures followed. Be sure to include enough detail so that someone else could repeat the experiment.
Write the results: In the results section, present your data in a clear and organized manner. Use tables, graphs, and figures to help illustrate your findings. Make sure to include any statistical analyses you performed.
Write the discussion: In the discussion section, interpret your results and explain what they mean. Discuss any patterns or trends you observed and explain how they relate to the research question. Compare your results to previous research in the field, and discuss any limitations or potential sources of error.
Write the conclusion: The conclusion should summarize the main findings of the experiment and explain their significance. You should also discuss any future directions for research in the field.
Proofread and revise: Once you have completed your first draft, proofread your report carefully to check for errors and inconsistencies. Revise your report as necessary to make sure it is clear, concise, and well-organized.
Read more about lab report here:
https://brainly.com/question/29500102
#SPJ1
ammonia is a weak electrolyte. which of the following is true about the behavior of ammonia in water? question 11 options: ammonia is insoluble in water ammonia forms no ions when it dissolves in water ammonia ionizes completely in water ammonia ionizes only partially in water
Answers
Ammonia ionizes only partially in water. Option 4 is correct.
When ammonia dissolves in water, it reacts with water to form ammonium ions (NH₄⁺) and hydroxide ions (OH⁻), according to the equation: NH₃ + H₂O ⇌ NH₄⁺ + OH⁻. However, this reaction is reversible and only a small fraction of ammonia molecules ionize to form ions. As a result, ammonia is classified as a weak electrolyte, meaning that it only conducts electricity weakly in solution.
Weak electrolytes are characterized by their partial ionization in solution, and they have relatively low electrical conductivity compared to strong electrolytes, which ionize completely in solution. Hence Option 4 is correct.
To learn more about Weak electrolytes, here
https://brainly.com/question/29771118
#SPJ4
If 120 cm3 of oxygen gas is collected at 27 oC and 713.3 mm Hg pressure, what will the volume (in cm3) of the dry gas be at STP?
Answers
If 120 cm³ of oxygen gas is collected at 713.3 mm Hg pressure, the volume of the dry gas at STP is 0.102 cm³.
How do you calculate the volume of the dry gas to be at STP?To solve this problem, we will use the ideal gas law, which relates the pressure, volume, temperature, and number of moles of a gas:
PV = nRT
First, we need to convert the given conditions to the correct units. The temperature is already in Celsius, so we need to convert it to kelvins by adding 273.15:
T = 27 + 273.15 = 300.15 K
The pressure is given in millimeters of mercury (mm Hg), so we need to convert it to atmospheres (atm) to use in the ideal gas law. There are 760 mm Hg in 1 atm, so:
P = 713.3 mm Hg / 760 mm Hg/atm = 0.938 atm
Next, we can use the ideal gas law to find the number of moles of oxygen gas:
n = PV/RT = (0.938 atm)(120 cm³)/(0.08206 L·atm/(mol·K))(300.15 K) = 0.00454 mol
Finally, we can use the molar volume of a gas at STP (standard temperature and pressure) to find the volume of the dry gas at STP. At STP, the temperature is 273.15 K and the pressure is 1 atm. The molar volume of a gas at STP is 22.4 L/mol, so:
V = n(22.4 L/mol) = (0.00454 mol)(22.4 L/mol) = 0.102 cm³
Therefore, the volume of the dry gas at STP is 0.102 cm³.
Learn more about ideal gas law here:
https://brainly.com/question/28257995
#SPJ1
Lila is a track and field athlete. She must complete four laps around a circular track. The track itself measures 400 meters from start to finish and the race took her 6 minutes to complete.
Which best describes her speed and velocity?
Her speed is 4. 4 m/s, and her velocity is 0 m/s.
Her speed is 1. 1 m/s, and her velocity is 0 m/s.
Her speed is 0 m/s, and her velocity is 2400 m/s.
Her speed is 4. 4 m/s, and her velocity is 4. 4 m/s
Answers
The best describes her speed and velocity is; Her speed is 4. 4 m/s, and her velocity will be 0 m/s. Option A is correct.
Lila's speed will be calculated by dividing the total distance she covered by time it took her to complete the race;
Speed = Total distance/Time
In this case, Lila will covered 4 laps, which is a total distance of 4 x 400 = 1600 meters. She completed the race in 6 minutes, which is 6 x 60 = 360 seconds. Therefore, her speed is;
Speed = 1600 meters / 360 seconds
Speed = 4.44 m/s (rounded to two decimal places)
Velocity, on the other hand, is a vector quantity that takes into account both speed and direction. Since Lila ran four laps around a circular track, she ended up at the same position where she started. However, her displacement (change in position) is zero, which means her velocity is also zero.
Hence, A. is the correct option.
To know more about velocity here
https://brainly.com/question/17127206
#SPJ4
--The given question is incomplete, the complete question is
"Lila is a track and field athlete. She must complete four laps around a circular track. The track itself measures 400 meters from start to finish and the race took her 6 minutes to complete. Which best describes her speed and velocity? A) Her speed is 4. 4 m/s, and her velocity is 0 m/s. B) Her speed is 1. 1 m/s, and her velocity is 0 m/s. C) Her speed is 0 m/s, and her velocity is 2400 m/s. D) Her speed is 4. 4 m/s, and her velocity is 4. 4 m/s"--
6Na + Fez0g -> 3NazO + 2Fe
If you are provided 200g of sodium and 250 grams of iron(Ill) oxide, how much of excess reagent is left?
Answers
The amount of excess reagent that will remain would be 11.76 g.
Stoichiometric problemTo determine the excess reagent in the reaction, we need to first determine which reactant is limiting and which reactant is in excess.
The balanced chemical equation for the reaction is:
6Na + Fe2O3 -> 3Na2O + 2Fe
The molar mass of Na is 23 g/mol, and the molar mass of Fe2O3 is 159.69 g/mol (2 x 55.85 g/mol for Fe + 3 x 16 g/mol for O).
Using the given masses, we can calculate the number of moles of each reactant:
Number of moles of Na = 200 g / 23 g/mol = 8.70 molNumber of moles of Fe2O3 = 250 g / 159.69 g/mol = 1.57 molAccording to the balanced chemical equation, 6 moles of Na react with 1 mole of Fe2O3. Therefore, the number of moles of Na required to react with 1.57 mol of Fe2O3 is:
(1.57 mol Fe2O3) x (6 mol Na/1 mol Fe2O3) = 9.42 mol Na
Since we only have 8.70 mol of Na available, it is the limiting reagent. This means that Fe2O3 is in excess.
To determine the amount of excess Fe2O3, we need to calculate how much Fe2O3 is required to react with 8.70 mol of Na:
(8.70 mol Na) x (1 mol Fe2O3/6 mol Na) x (159.69 g/mol Fe2O3) = 238.24 g Fe2O3
Since we only have 250 g of Fe2O3, the amount of excess Fe2O3 is:
250 g - 238.24 g = 11.76 g
Therefore, the amount of excess Fe2O3 left after the reaction is 11.76 g.
More on stoichiometry can be found here: https://brainly.com/question/29775083
#SPJ1
PLEASE ANSWER ASAP
1. How many atoms are present in 8.500 mole of chlorine atoms?
2. Determine the mass (g) of 15.50 mole of oxygen.
3. Determine the number of moles of helium in 1.953 x 108 g of helium.
4. Calculate the number of atoms in 147.82 g of sulfur.
5. Determine the molar mass of Co.
6. Determine the formula mass of Ca3(PO4)2.
IT WOULD BE HELPFUL
Answers
1) 5.1167 x 10²⁴atoms of chlorine. 2) 248.00 g. 3) 4.8825 x 10⁷ moles of helium. 4) 2.7757 x 10²⁴ atoms of sulfur. 5) Molar mass of Co (cobalt) is 58.93 g/mol. 6) Formula mass = 310.18 g/mol.
What is meant by formula mass?Sum of the atomic masses of all the atoms in chemical formula is called formula mass
1.) Number of atoms = 8.500 moles x 6.022 x 10²³ atoms/mole = 5.1167 x 10²⁴ atoms of chlorine.
2.) Molar mass of oxygen is 16.00 g/mol. Therefore:
Mass of 15.50 moles of oxygen = 15.50 moles x 16.00 g/mol = 248.00 g.
3.) Molar mass of helium is 4.00 g/mol. Therefore, the number of moles of helium in 1.953 x 10⁸ g is:
Number of moles = 1.953 x 10⁸ g / 4.00 g/mol = 4.8825 x 10⁷ moles of helium.
4.) Molar mass of sulfur is 32.06 g/mol. Therefore, the number of moles of sulfur in 147.82 g is:
Number of moles = 147.82 g / 32.06 g/mol = 4.6084 moles of sulfur.
To find the number of atoms, we can use Avogadro's number again:
Number of atoms = 4.6084 moles x 6.022 x 10²³ atoms/mole = 2.7757 x 10²⁴ atoms of sulfur.
5.) Molar mass of Co (cobalt) is 58.93 g/mol.
6.) Ca₃(PO₄)₂ contains 3 calcium atoms, 2 phosphorus atoms, and 8 oxygen atoms.
Atomic masses of these elements are:
Calcium (Ca) = 40.08 g/mol
Phosphorus (P) = 30.97 g/mol
Oxygen (O) = 16.00 g/mol
Therefore, formula mass of Ca₃(PO₄)₂ is:
Formula mass = (3 x 40.08 g/mol) + (2 x 30.97 g/mol) + (8 x 16.00 g/mol)
= 120.24 g/mol + 61.94 g/mol + 128.00 g/mol
= 310.18 g/mol.
To know more about formula mass, refer
https://brainly.com/question/21334167
#SPJ1
given two orbitals as linear combinations of two atomic orbitals on carbon atom in ethene: where the hydrogen-like atomic orbitals are orthonormal. what is the value of the overlap integra
Answers
the overlap integral simplifies to:
S = c1c2 + d1d2d1d2.
To calculate the overlap integral between two linear combinations of atomic orbitals on a carbon atom in ethene, we first need to express the orbitals in terms of the hydrogen-like atomic orbitals. Let's assume that the two orbitals are denoted as ψ1 and ψ2, and can be expressed as linear combinations of the hydrogen-like atomic orbitals ϕ1 and ϕ2 as follows:
ψ1 = c1ϕ1 + d1ϕ2
ψ2 = c2ϕ1 + d2ϕ2
where c1, d1, c2, and d2 are constants.
The overlap integral between these two orbitals can be calculated using the following formula:
S = ∫ψ1ψ2*dτ
where dτ represents the infinitesimal volume element.
Substituting for ψ1 and ψ2, we get:
S = ∫(c1ϕ1 + d1ϕ2)(c2ϕ1 + d2ϕ2)*dτ
Expanding the product, we get:
S = c1c2∫ϕ1ϕ1*dτ + c1d2∫ϕ1ϕ2*dτ + d1c2∫ϕ2ϕ1*dτ + d1d2∫ϕ2ϕ2*dτ
Since the hydrogen-like atomic orbitals are orthonormal, the integral of ϕ1ϕ2 and ϕ2ϕ1 will be zero. Therefore, we can simplify the expression as follows:
S = c1c2∫ϕ1ϕ1*dτ + d1d2∫ϕ2ϕ2*dτ
Using the orthonormality of the hydrogen-like atomic orbitals, we know that the integral of ϕ1ϕ1 and ϕ2ϕ2 will both be equal to 1. Therefore, the overlap integral simplifies to:
S = c1c2 + d1d2d1d2.
In order to calculate the value of S, we need to know the values of the constants c1, d1, c2, and d2. These constants will depend on the specific linear combinations of atomic orbitals that we are considering. Without this information, we cannot calculate the value of the overlap integral.
Visit to know more about Integral:-
brainly.com/question/22008756
#SPJ11
If two orbitals as linear combinations of two atomic orbitals on carbon atom in ethene, then value of the overlap integral [tex]S_{12} = \int{\phi_{1}}^{*}\phi_{2}d \tau[/tex], is equals to zero. So, option(b) is correct.
Orthonormal atomic orbitals are follow the following property:
[tex]\int{ m _i }* n_i d\tau = 1[/tex][tex]\int m_i^{*} n_j d\tau = 0[/tex]Now, we have provide that two orbitals are as a linear combinations of two atomic orbitals on carbon atom in ethene. [tex]\phi_{1 } = \frac{1}{ \sqrt{2} } ( {\psi_{2s }} + {\psi_{2p }}_{2})[/tex]
[tex]\phi_{2 } = \frac{1}{ \sqrt{2} } ( \psi_{2 s} - {\psi_{2p} }_{2})[/tex]
In the ethylene molecule, consists each carbon atom is bonded to two hydrogen atoms. Therefore, for the C-H, σ bond (sp²(C) - 1s(H)) in ethylene, the two sp² hybrid orbitals overlap with the 1s orbitals of the two hydrogen atoms. Let the hydrogen-like atomic orbitals, [tex]\psi_{2 s} and {\psi_{2p} }_{2}[/tex] are orthonormal to each other. So, the overlap integral [tex]S_{12} = \int{ \phi_{1}}^{*}\phi_{2}d \tau[/tex]
[tex] = \int \frac{1}{\sqrt{2}}( \psi_{2s} + {\psi_{2p} }_{2}) \frac{1}{\sqrt{2}}( \psi_{2s} - {\psi_{2p} }_{2})d \tau\\ [/tex]
[tex] = \frac{1}{\sqrt{2}}( \int \psi_{2s}\psi_{2s} d \tau + \int {\psi_{2p} }_{2}\psi_{2s} d \tau - \int \psi_{2s} {\psi_{2p}}_{2} d \tau - \int {\psi_{2p} }_{2} {\psi_{2p} }_{2} d \tau) \\ [/tex].
Using above formula, [tex]\psi_{2 s} [/tex] and [tex]{\psi_{2p} }_{2}[/tex] are orthonormal so, [tex]\int \psi_{2 s} {\psi_{2p} }_{2} d\tau = 0[/tex]. Also [tex]\psi_{2 s} [/tex] and [tex]\psi_{2 s}[/tex] are normalised so [tex]\int \psi_{2 s} \psi_{2 s} d\tau = 1[/tex]. Similarly [tex]\int {\psi_{2p} }_{2} {\psi_{2p} }_{2} d\tau = 1 [/tex].
Substitute all integral values in equation (1),
= 1 + 0 - 0 - 1
= 0
Hence, the required integral value is 0.
For more information about atomic orbitals, visit :
https://brainly.com/question/30911211
#SPJ4
Complete question:
given two orbitals as linear combinations of two atomic orbitals on carbon atom in ethene:
[tex]\phi_{1 } = \frac{1}{ \sqrt{2} } ( {\psi_{2s }} + {\psi_{2p }}_{2})[/tex]
[tex]\phi_{2 } = \frac{1}{ \sqrt{2} } ( \psi_{2 s} - {\psi_{2p} }_{2})[/tex]
where the hydrogen-like atomic orbitals are orthonormal. what is the value of the overlap integral,
[tex] S_{12} = \int \phi_{1} \times \phi_{2}dr[/tex]
a) 1
b) 0
c) 1.5
d) 2
Calculate the pH of a solution that contains 52. mL of 0.428 M HCl, and 44.5
mL of 0.500 M methylamine, CH3NH₂. The pKb, of methylamine is 3.34.
Answers
Answer:
Explanation:
The pH of the solution is 10.80
The pH of the solution is 10.80.
Explanation: This can be calculated using the Henderson-Hasselbalch equation, which takes into account the acid dissociation constant (pKa) of the acid and the concentration of the acid and its conjugate base. The HCl dissociates completely in water, so it does not affect the pH calculation.
The methylamine acts as a weak base and reacts with water to form its conjugate acid, which determines the pH of the solution.
The pKb of methylamine is used to calculate its pKa, which is then used in the Henderson-Hasselbalch equation.
Refer to this link to know more about how to calculate the pH of a solution
https://brainly.com/question/30881040?referrer=searchResults
Please help!!!! Quick please!!
Answers
4. The number of each Race Car Part present in Container A are:
Body (B) - 3Cylinder (Cy) - 10Engine (E) - 2Tire (Tr) - 9How to determine number of race cars?5. To draw the maximum number of cars that can be made from the parts in Container A:
Each car requires 1 Body (B), 4 Tires (Tr), 1 Engine (E), and 2 Cylinders (Cy).
We have 3 Bodies (B), 10 Cylinders (Cy), 2 Engines (E), and 9 Tires (Tr).
The limiting parts are the Engines (E) and the Cylinders (Cy), since we don't have enough of either part to build more than 2 cars.
Therefore, we can build a maximum of 2 complete cars from the parts in Container A, and we will have excess parts remaining:
1 Body (B)
6 Tires (Tr)
0 Engines (E)
6 Cylinders (Cy)
6. The student is incorrect because although there are 3 car bodies in Container A, we also need 4 tires, 1 engine, and 2 cylinders for each car. We don't have enough engines or cylinders to build 3 complete cars, so the number of bodies is not the limiting factor.
7. a. To determine the number of complete cars that can be built:
Each car requires 1 Body (B), 4 Tires (Tr), 1 Engine (E), and 2 Cylinders (Cy).
We have a large number of Bodies (B) and Tires (Tr), so we don't need to worry about those parts.
We only have 5 Engines (E) and 12 Cylinders (Cy).
The limiting part is the Cylinders (Cy), since each car requires 2 cylinders and we only have 12.
Therefore, we can build a maximum of 6 complete cars with the available parts:
6 Bodies (B)
24 Tires (Tr)
5 Engines (E)
12 Cylinders (Cy)
b. The limiting part is the Cylinders (Cy), since we only have enough cylinders to build 6 cars, but we have enough engines to build 5 times as many cars.
Find out more on limiting reagents here: https://brainly.com/question/19033878
#SPJ1
hydroboration of terminal alkynes yieldgroup of answer choicesa. methyl ketoneb.ketonec.aldehyded.carboxylic acid
Answers
Hydroboration of terminal alkynes yields aldehydes.
Here's a step-by-step explanation:
1. Terminal alkynes are reacted with a borane reagent (such as BH₃) in a process called hydroboration.
2. During hydroboration, the borane adds across the triple bond of the terminal alkyne in a syn addition, resulting in the formation of an organoborane intermediate.
3. The organoborane intermediate is then oxidized with hydrogen peroxide (H₂O₂) and a hydroxide ion (OH⁻) in a process called oxidation.
4. This oxidation step converts the organoborane intermediate into an aldehyde functional group.
So, the correct answer is (c) aldehyde.
Learn more about hydroboration at https://brainly.com/question/11655454
#SPJ11
The hydroboration of terminal alkynes yields an aldehyde. During the hydroboration process
boron and hydrogen are added across the carbon-carbon triple bond of the alkyne, which yields an unstable intermediate. This intermediate quickly reacts with water to form an aldehyde.
It is important to note that the product of the hydroboration of an alkyne can be influenced by the reaction conditions and the substituents present on the alkyne. However, for a terminal alkyne, the product is typically an aldehyde.
Learn more about hydroboration here:
https://brainly.com/question/31324581
#SPJ11
a 1.25 g sample of co2 is contained in a 750. ml flask at 22.5 c. what is the pressure of the gas, in atm?
Answers
The pressure of gas is 1.05 atm when a 1.25 g sample of CO₂ is contained in a 750ml flask at 22.5°C.
Molecular weight of CO₂ is 1.25g ,Volume of CO₂ is 750ml,Temperature of CO₂ is 22.5°C and the gas constant is 0.08206 L atm/mol K.
Using the ideal gas law equation the pressure is found to be 1.05 atm.
To calculate the pressure of the gas, we can use the ideal gas law equation: [tex]PV=nRT[/tex]
Where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin.
First, we need to convert the volume to liters by dividing by 1000: 750 ml = 0.75 L.
Next, we need to calculate the number of moles of CO₂ present in the flask. We can use the molecular weight of CO₂ to convert from grams to moles:
[tex]1.25 * (1 /44.01 ) = 0.0284 mol[/tex]
Now we can plug in the values into the ideal gas law equation:
[tex]PV=nRT[/tex]
[tex]P * 0.75 L = 0.0284 mol * 0.08206 L*atm/mol*K * (22.5 + 273.15) K[/tex]
Simplifying and solving for P, we get:
[tex]P = (0.0284 * 0.08206 * 295.65) / 0.75 = 1.05 atm[/tex]
Therefore, the pressure of the gas in the flask is 1.05 atm.
Learn more about ideal gas law equation here:
https://brainly.com/question/15379358
#SPJ11
A hammer and a feather are dropped from the same height by an astronaut on a planet without air. How will their falls compare?
Answers
The hammer and the feather are dropped from same height by the astronaut on the planet without the air. The feather will fell at the same rate as the the hammer.
The hammer and the feather are dropped from equal height by the astronaut on the planet without the air. They were the essentially in the vacuum, and there was the no air resistance and because of the feather will fell at the same rate as compared to the hammer, the Galileo had to concluded that the hundreds of the years before.
All the objects that released together will fall at the same rate excluding the factor of the mass.
To learn more about astronaut here
https://brainly.com/question/16843793
#SPJ4
what mass of calcium metal is produced when molten caf2 is electrolyzed by a current of 9.55 a for 19 h? 136 g
Answers
when molten CaF2 is electrolyzed by a current of 9.55 A for 19 h, approximately 136 g of calcium metal is produced.
To determine the mass of calcium produced when molten CaF2 is electrolyzed by a current of 9.55 A for 19 h, we'll use Faraday's Law of Electrolysis.
First, calculate the total charge passed through the electrolyte:
Charge (Q) = Current (I) × Time (t)
Q = 9.55 A × (19 h × 3600 s/h) = 653,940 C
Next, determine the number of moles of electrons (n):
n = Q / (Faraday constant F)
n = 653,940 C / (96,485 C/mol) ≈ 6.77 mol
The balanced equation for the electrolysis of CaF2 is:
2F- → F2 + 2e-
Ca2+ + 2e- → Ca
The mole ratio between calcium and electrons is 1:2. So, the number of moles of calcium produced is:
Moles of Ca = 0.5 × Moles of electrons
Moles of Ca = 0.5 × 6.77 mol ≈ 3.39 mol
Finally, calculate the mass of calcium:
Mass of Ca = Moles of Ca × Molar mass of Ca
Mass of Ca = 3.39 mol × 40.08 g/mol ≈ 136 g
To learn more about : calcium
https://brainly.com/question/29231164
#SPJ11