Answers
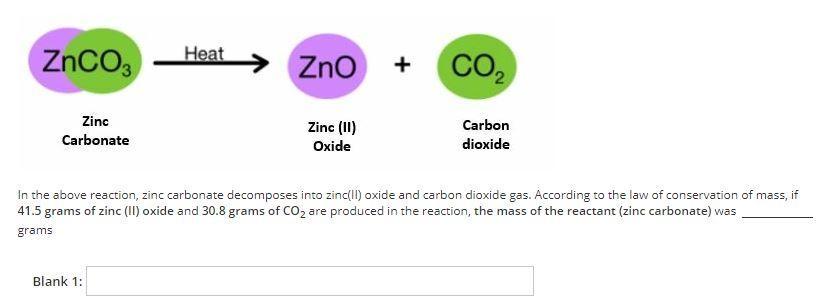
72.3 grams. This can be calculated by adding the mass of the products (41.5 + 30.8 = 72.3).
What is mass?Mass is a fundamental concept in physics that defines the measure of a body's resistance to acceleration, or the amount of matter that an object contains. It is measured in standard SI units of kilograms (kg) or pounds (lb). Mass is distinct from weight, which is the measure of the force of gravity acting on an object. Mass is not affected by gravity, whereas weight is. Mass is an intrinsic property of an object, whereas weight is a force that changes depending on the location of the object, so it is only meaningful in a gravitational field. The mass of an object is constant, no matter where it is located in the universe.
This is according to the law of conservation of mass, which states that in a chemical reaction, matter is neither created nor destroyed, so the mass of the reactant must equal the mass of the products.
To learn more about mass
https://brainly.com/question/24191825
#SPJ1
Related Questions
I need help please help me please
Answers
Answer: compound thing(picture attached)
Bronze is an alloy made by combining copper and tin. The exact composition of bronze can vary depending on the desired properties, but generally, bronze contains anywhere from 5% to 25% tin.
The reasons why the first humans experimented with making bronze are not fully known, but it is believed that they discovered that adding tin to copper improved its properties, making it harder, more durable, and easier to cast. This would have made it more suitable for weapons, tools, and other objects.
Bronze provided several benefits to early humans. Firstly, bronze tools and weapons were much more durable than those made of pure copper or stone. This made it easier for early humans to hunt, farm, and build, and allowed them to produce more sophisticated and efficient tools. Additionally, bronze objects were more aesthetically pleasing and could be used for decorative purposes. Bronze also played an important role in early trade, as it could be used as a form of currency and was highly valued by many cultures.
In summary, bronze was an important technological advancement in early human history, and its discovery and use played a significant role in the development of human civilization.
Explanation:
Which of the following correctly defines work? Responses the amount of power consumed per unit time by an object the amount of power consumed per unit time by an object the amount of force exerted per unit time in order to accelerate an object the amount of force exerted per unit time in order to accelerate an object a net force applied through a distance in order to displace an object a net force applied through a distance in order to displace an object the amount of work done per unit time on an object the amount of work done per unit time on an object
Answers
The correct definition of work is: net force applied through a distance in order to displace an object.
What is work?In physics, work is defined as the energy transferred to or from any object by means of force acting on the object as it moves through displacement.
More specifically, work is calculated as the product of force acting on an object and distance the object is displaced, multiplied by cosine of the angle between the force and displacement. Mathematically, work can be expressed as W = Fd cos(theta), where W is work, F is the force, d is displacement, and theta is angle between the force and displacement vectors.
To know more about work, refer
https://brainly.com/question/28356414
#SPJ1
if you can fill out this worksheet 100 pts! only 5 questions, about stoichiometry PLEASE HELP ASAP!!
Answers
Given: NaOH, H₂SO₄. Wanted: Na₂SO₄.
Percent yield = (325 g / 355.1 g) × 100 = 91.5%
molar mass of Na₂SO₄ is 142.04 g/mol.
The mole ratio needed is 2:1 (two moles of NaOH react with one mole of H₂SO₄ to produce one mole of Na₂SO₄).
The molar mass of Na₂SO₄ is 142.04 g/mol.
To determine the theoretical yield, we need to first calculate the limiting reagent.
Using the mole ratio, we can calculate the number of moles of H₂SO₄ required to react with 5.00 moles of NaOH:
5.00 mol NaOH × (1 mol H₂SO₄ / 2 mol NaOH) = 2.50 mol H₂SO₄
Since we have 7.00 moles of H₂SO₄, it is in excess and NaOH is the limiting reagent.
The number of moles of Na₂SO₄ that can be produced is:
5.00 mol NaOH × (1 mol Na₂SO₄ / 2 mol NaOH) = 2.50 mol Na₂SO₄
The theoretical yield of Na₂SO₄ is:
2.50 mol Na₂SO₄ × 142.04 g/mol = 355.1 g Na₂SO₄
The percent yield is calculated by dividing the actual yield (325 g) by the theoretical yield (355.1 g) and multiplying by 100:
Percent yield = (325 g / 355.1 g) × 100 = 91.5%
learn more about stoichiometry here
https://brainly.com/question/16060223
#SPJ1
Which techniques would be best for separating a colloid mixture but would not work well with solutions? Check all that apply.
distillation
centrifugation
boiling/heating
chromatography
crystallization
long standing
Answers
Centrifugation, chromatography, and long standing are the procedures that would work well for separating a colloid mixture but would not be effective with solutions.
Can evaporation be used to separate colloids?Stratification that is "inverted" is created when the larger colloids are forced to the bottom. The reason for this qualitative segregation is that evaporation causes a local rise in colloid concentration close to the film-air contact, which results in a chemical potential gradient for both colloid species.
Which technique is better for purifying colloidal solution?Dialysis: The removal of ions from a solution through the diffusion process through a permeable membrane is known as dialysis. This procedure involves filling a permeable membrane bag with a sol made up of ions or molecules and submerging it in water.
To know more about chromatography visit:-
https://brainly.com/question/29485560
#SPJ1
Answer:
centrifugation
boiling/heating
long standing
Explanation: I just got it right
(a) Does the lattice energy of an ionic solid increase or decrease (i) as the charges of the ions increase as the sizes of the ions increase? (b) Arrange the following substances not listed in Table 8.1 according to their expected lattice energies, listing them from lowest lattice energy to the highest: MgS, KI, GaN, LiBr.
Answers
(a) With charge and size increase, lattice energy of ionic solid increases. (b) KI (low charges, large ions) < LiBr (low charges, medium-sized ions) < MgS (high charges, medium-sized ions) < GaN (very high charges, small ions)
(a) The lattice energy of an ionic solid depends on two factors: the charges of the ions and the sizes of the ions.
(i) As the charges of the ions increase, the lattice energy of an ionic solid increases. This is because the electrostatic attraction between the ions becomes stronger with higher charges, leading to a more stable and higher-energy lattice.
(ii) As the sizes of the ions increase, the lattice energy of an ionic solid decreases. Larger ions have a greater distance between their positive and negative charges, which weakens the electrostatic attraction between them and results in a lower-energy lattice.
(b) To arrange the substances according to their expected lattice energies, consider the charges and sizes of the ions:
MgS: Mg²⁺ and S²⁻ - high charges, medium-sized ions
KI: K⁺ and I⁻ - low charges, large ions
GaN: Ga³⁺ and N³⁻ - very high charges, small ions
LiBr: Li⁺ and Br⁻ - low charges, medium-sized ions
Based on this information, the substances can be arranged as follows (from lowest lattice energy to highest):
KI (low charges, large ions) < LiBr (low charges, medium-sized ions) < MgS (high charges, medium-sized ions) < GaN (very high charges, small ions)
Learn more about lattice energy here:
https://brainly.com/question/18222315
#SPJ11
(a) The lattice energy of an ionic solid generally increases as the charges of the ions increase and/or as the sizes of the ions decrease.
(b) The substances arranged according to their expected lattice energies from lowest to highest are: KI < LiBr < MgS < GaN.
What are the factors affecting Lattice Energy?(a) The lattice energy of an ionic solid:
(i) Increases as the charges of the ions increase, because the electrostatic force between the ions becomes stronger, leading to a more stable lattice.
(ii) Decreases as the sizes of the ions increase, because the distance between the ions increases, which results in a weaker electrostatic force and lower lattice energy.
(b) To arrange the following substances according to their expected lattice energies from lowest to highest, we need to consider both the charges and the sizes of the ions:
1. KI (large ions, lower charges): K⁺ has a +1 charge, and I⁻ has a -1 charge. Both ions are relatively large.
2. LiBr (smaller ions, lower charges): Li⁺ has a +1 charge, and Br⁻ has a -1 charge. Both ions are smaller than K⁺ and I⁻.
3. MgS (smaller ions, higher charges): Mg²⁺ has a +2 charge, and S²⁻ has a -2 charge. Both ions are smaller than K⁺ and I⁻, and their charges are higher than LiBr.
4. GaN (small ions, higher charges): Ga³⁺ has a +3 charge, and N³⁻ has a -3 charge. Both ions are small, and their charges are the highest among the listed substances.
To know more about Lattice energy:
https://brainly.com/question/31535671
#SPJ11
a sample of 35.1 g of methane gas has a volume of 2.55 l at a pressure of 2.70 atm. calculate the temperature.
Answers
A sample of 35.1 g of methane gas has a volume of 2.55 l at a pressure of 2.70 atm. The temperature of the sample of methane gas is 224.8 K.
The temperature of the sample of methane gas can be calculated using the ideal gas law equation, PV = nRT, where P is the pressure in atmospheres, V is the volume in liters, n is the amount of gas in moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Since the pressure and volume are given, we can calculate the moles of methane gas using the relationship n= PV/RT.
Plugging in the given values, n = (2.7 atm)(2.55 L)/(0.08206 L·atm/mol·K)(T) = 0.824 mol.
Then, rearranging the ideal gas law equation, T = PV/nR, and plugging in our values, T = (2.7 atm)(2.55 L)/(0.824 mol)(0.08206 L·atm/mol·K) = 224.8 K.
As a result, the sample of methane gas had a temperature of 224.8 K.
To learn more about methane gas visit:
https://brainly.com/question/23151003
#SPJ4
superficial frostbite is a blank and results in blank
Answers
Superficial frostbite is a second-degree frostbite (a type of injury) and results in clear skin blisters.
Frostbite is damage of skin due to cold temperatures. The victim of frostbite is mostly unaware of it because a frozen tissue is numb. It can be cured but depends upon the stages of frostbite. There are three stages of frostbite as given below:
First stage is Frostnip, cause loss of feeling in skin occurs. Skin color becomes red and purple.
Second stage is Superficial Frostbite, cause clear skin blisters. Skin color changes from red to paler. A fluid-filled blister may appear 24 to 36 hours after color changing of skin
Third stage is Deep Frostbite, cause joints or muscles no longer work. Skin color changes to black and the area turns hard.
Redness or pain in any skin area maybe the first sign of frostbite.
Thus, when weather is very cold, stay indoors or dress in layers to prevent serious health problems.
Learn more about Frostbite here:
brainly.com/question/14460475
#SPJ11
Superficial frostbite is a type of frostbite that affects the outer layers of the skin and results in localized damage to the skin and underlying tissues. It is considered a mild form of frostbite and usually affects the fingers, toes, ears, nose, and cheeks.
The symptoms of superficial frostbite can include numbness, tingling, stinging, and burning sensations in the affected area. The skin may also appear pale or waxy and may be hard to the touch. In some cases, blisters may form several hours after rewarming the affected area.
If treated promptly and properly, superficial frostbite usually heals without complications. However, if left untreated, it can progress to deeper layers of tissue, leading to more severe frostbite and potential tissue damage.
For more question on Superficial frostbite click on
https://brainly.com/question/31453309
#SPJ11
given the equation3cl2 8nh3 =n2 6nh$cl how many moles of nh3 are required to produce 12 moles of nh4cl
Answers
16 moles of NH3 are required to produce 12 moles of NH4Cl.
Given the balanced equation:
3Cl2 + 8NH3 → N2 + 6NH4Cl
To determine how many moles of NH3 are required to produce 12 moles of NH4Cl, we can use the stoichiometry of the equation. We can see that 6 moles of NH4Cl are produced from 8 moles of NH3.
Follow these steps:
1. Write down the balanced equation:
3Cl2 + 8NH3 → N2 + 6NH4Cl
2. Determine the stoichiometric ratio between NH3 and NH4Cl:
8 moles of NH3 : 6 moles of NH4Cl
3. Calculate the moles of NH3 needed to produce 12 moles of NH4Cl using the stoichiometric ratio:
(8 moles of NH3 / 6 moles of NH4Cl) * 12 moles of NH4Cl = 16 moles of NH3
16 moles of NH3 are required to produce 12 moles of NH4Cl.
Learn more about moles here:
https://brainly.com/question/15833820
#SPJ11
Given the equation 3[tex]Cl_{2}[/tex] + 8[tex]NH_{3}[/tex] = [tex]N_{2}[/tex] + 6 [tex]NH_{4}Cl[/tex], 16 moles of [tex]NH_{3}[/tex] are required to produce 12 moles of [tex]NH_{4}Cl[/tex].
How to determine the number of moles?To know how many moles of [tex]NH_{3}[/tex] are required to produce 12 moles of [tex]NH_{4}Cl[/tex], we can follow the steps below:
Step 1: Determine the mole ratio between [tex]NH_{3}[/tex] and [tex]NH_{4}Cl[/tex] from the balanced equation. In this case, it is 8 moles of [tex]NH_{3}[/tex] to 6 moles of [tex]NH_{4}Cl[/tex].
Step 2: Set up a proportion to find the moles of NH3 needed for 12 moles of [tex]NH_{4}Cl[/tex]:
(8 moles [tex]NH_{3}[/tex] / 6 moles [tex]NH_{4}Cl[/tex]) = (x moles [tex]NH_{3}[/tex] / 12 moles [tex]NH_{4}Cl[/tex])
Step 3: Solve for x:
x moles [tex]NH_{3}[/tex] = (8 moles [tex]NH_{3}[/tex] / 6 moles [tex]NH_{4}Cl[/tex]) * 12 moles [tex]NH_{4}Cl[/tex]
Step 4: Calculate x:
x moles [tex]NH_{3}[/tex] = (8/6) * 12 = 16 moles [tex]NH_{3}[/tex]
To know more about Stoichiometry:
https://brainly.com/question/29195098
#SPJ11
How does temperature affect ocean currents?
Answers
50.0 ml of 0.10 m hcl is mixed with 50.0 ml of 0.10 m naoh. the solution temperature rises by 3.0 calculate the enthalpy
Answers
To calculate the enthalpy of the reaction, we need to use the equation:
q = mCΔT where q is the heat absorbed or released by the reaction, m is the mass of the solution , C is the specific heat capacity of the solution.
First, we need to calculate the amount of heat absorbed or released by the reaction. Since the reaction is exothermic (it releases heat), q will be negative. We can use the following equation to calculate q:
q = -CΔT
q = -(100 g)(4.18 J/g°C)(3.0°C) = -1254 J
Now we can use the following equation to calculate the enthalpy of the reaction (ΔH):
ΔH = q/n
where n is the number of moles of limiting reactant (in this case, either HCl or NaOH).
To find the number of moles of HCl, we can use the following equation:
n = C × V
where C is the concentration of HCl (0.10 M) and V is the volume of HCl (50.0 mL = 0.050 L).
n = (0.10 M)(0.050 L) = 0.0050 moles
To find the number of moles of NaOH, we can use the same equation:
n = C × V
where C is the concentration of NaOH (0.10 M) and V is the volume of NaOH (50.0 mL = 0.050 L).
n = (0.10 M)(0.050 L) = 0.0050 moles
Since the stoichiometric ratio between HCl and NaOH is 1:1, the number of moles of HCl and NaOH are equal. Therefore, we can use either value for n in the equation for ΔH.
ΔH = -1254 J / 0.0050 moles
ΔH = -250800 J/mol
Therefore, the enthalpy of the reaction is -250.8 kJ/mol.
Learn more about absorbed here
https://brainly.com/question/6838193
#SPJ11
the gradual increase or decrease in concentration from one point to another constitutes a concentration
Answers
The gradual increase or decrease in concentration from one point to another constitutes a concentration gradient. This gradient can occur within a single substance, such as a solution or gas, or between different substances in a system.
Concentration gradients play an important role in various natural and artificial processes, including diffusion, osmosis, and chemical reactions. A concentration gradient is the change in the concentration of a substance over a distance. It often results in the passive or active movement of particles from areas of high concentration to areas of low concentration, a process known as diffusion or transport.
The direction and magnitude of the concentration gradient can influence the rate and direction of these processes, making it a critical parameter to consider in many scientific and engineering applications.
To know more about diffusion:
https://brainly.com/question/30697046
#SPJ11
Yes, the gradual increase or decrease in the amount or density of a substance from one point to another is referred to as a concentration gradient. This can occur in various settings, such as in chemical reactions or in the distribution of molecules within a cell or organism. The concept of concentration is essential in understanding many biological and chemical processes, as it helps to determine how different substances interact and affect one another.
Concentration gradients are important in a wide range of biological, chemical, and physical processes. For example, in the human body, concentration gradients of ions and other molecules are essential for the functioning of cells and tissues. In addition, concentration gradients can drive the diffusion of gases, the movement of water in and out of cells, and many other important biological processes.
Overall, the gradual increase or decrease in concentration from one point to another constitutes a concentration gradient, which is a fundamental concept in many areas of science and engineering.
Learn more about concentration gradient here:
https://brainly.com/question/11391123
#SPJ11
which statements are true? a reducing agent gains electrons. zn2 zn 2 is formed from the oxidation of zn(s) zn ( s ) . an oxidizing agent gains electrons. na na is formed from the reduction of na(s) na ( s ) . the oxidation number for cu(s) cu ( s ) is 2. the oxidation number for hg(l) hg ( l ) is 0.
Answers
The true statements are: a reducing agent gains electrons, Na⁺ is formed from the reduction of Na(s), the oxidation number for Cu(s) is +2, and the oxidation number for Hg(l) is 0, the correct options are 1, 4, 5, and 6.
A reducing agent is a substance that causes reduction by providing electrons to another substance. Thus, a reducing agent gains electrons. Sodium metal (Na) is reduced to form Na⁺ ions by losing one electron. The oxidation state of Na changes from 0 to +1, indicating the loss of one electron.
Copper metal (Cu) has an oxidation state of 0 because it is in its elemental form. However, Cu²⁺ ion has an oxidation state of +2 because it has lost two electrons. The oxidation state of mercury (Hg) in its elemental form (liquid) is 0 because each atom has an equal number of protons and electrons, the correct options are 1, 4, 5, and 6.
To learn more about electrons follow the link:
https://brainly.com/question/1255220
#SPJ4
The complete question is:
Which statements are true?
1 a reducing agent gains electrons
2 Zn²⁺ is formed from the oxidation of Zn(s)
3 an oxidizing agent gains electrons
4 Na⁺ is formed from the reduction of Na(s)
5 the oxidation number for Cu(s) is +2
6 the oxidation number for Hg(l) is 0
Silver nitrate and iron (III) chloride are reacted. 27.0 g silver nitrate and 43.5 g iron (III) chloride are used in the reaction.
3 AgNO3 + FeCl3 --> 3 AgCl + Fe(NO3)3
1. Using the limiting reactant, calculate how many grams of silver chloride are produced.
Answers
The mass of silver chloride produced is 7.24 grams. To determine the limiting reactant,
we need to calculate the amount of product that each reactant would produce if reacted completely, and the reactant that produces the least amount of product will be the limiting reactant.
First, we need to write the balanced chemical equation for the reaction:
3 AgNO₃ + FeCl₃ --> 3 AgCl + Fe(NO₃)³
The molar mass of AgNO₃ is 169.87 g/mol (107.87 g/mol for Ag, 14.01 g/mol for N, and 3 x 16.00 g/mol for 3 O atoms). The molar mass of FeCl₃ is 162.20 g/mol (55.85 g/mol for Fe and 3 x 35.45 g/mol for 3 Cl atoms).
Using the given masses, we can calculate the number of moles of each reactant:
Number of moles of AgNO₃ = 27.0 g / 169.87 g/mol = 0.159 moles
Number of moles of FeCl₃ = 43.5 g / 162.20 g/mol = 0.268 moles
According to the balanced equation, 3 moles of AgNO₃ react with 1 mole of FeCl₃ to produce 3 moles of AgCl. Therefore, if all the AgNO₃ were to react, we would expect to produce:
3 moles AgCl / 3 moles AgNO₃ x 0.159 moles AgNO₃ = 0.159 moles AgCl
Similarly, if all the FeCl₃ were to react, we would expect to produce:
1 mole AgCl / 1 mole FeCl₃ x 0.268 moles FeCl₃ = 0.268 moles AgCl
Since the calculated amount of AgCl from AgNO₃ is smaller than that from FeCl₃, AgNO₃ is the limiting reactant. Therefore, we can calculate the amount of AgCl produced based on the moles of AgNO₃:
1 mole AgCl / 3 moles AgNO₃ x 0.159 moles AgNO₃ x 143.32 g/mol AgCl = 7.24 g AgCl
Therefore, the mass of silver chloride produced is 7.24 grams.
To know more about silver chloride, visit:
https://brainly.com/question/12912140
#SPJ1
a solution contains 0.50 m acetic acid () and 0.50 m sodium acetate (). what are the major species in this solution?
Answers
The major species present in the given solution are acetic acid (CH3COOH), sodium ions (Na+), acetate ions (CH3COO-), and water (H2O).
The sodium acetate and acetic acid ions will be present in the solution in equal amounts because the solution includes 0.50 m of acetic acid and 0.50 m of sodium acetate.
Because it is a weak acid, acetic acid will partially dissociate in the solution to produce hydrogen ions (H+) and acetate ions.
The sodium acetate, on the other hand, will totally dissociate into sodium ions and acetate ions.
As a result, the acetic acid, sodium ions, acetate ions, and water are the main species in the solution, with the acetate ions being the most prevalent species.
Complete Question:
A solution contains 0.50 m of acetic acid (CH3COOH) and 0.50 m of sodium acetate (CH3COONa). What are the major species in this solution?
To learn more about acetic acid visit:
https://brainly.com/question/15231908
#SPJ4
What is a likely purpose of the hair in an adult’s armpits and genital regions, especially given that this hair grows during puberty?
Think about an animal like a rhinoceros, a deer, or an antelope. What parts of their body other than their hair must be composed of quite similar material to your nails and hair?
What kind of locations in the world (either in the United States or globally) might be easier to live in for people with Seasonal Affective Disorder? Which kinds of places might be worse?
Your friend Olivia has a blemish on her shoulder that she can’t easily see herself, so she asks you to check it out for her to help her decide if she should see her doctor. What are at least three things you would look for to help you advise her? (Remember: ABCDE!)
What might an elevated skin temperature indicate beside a fever from a cold, flu, or other typical viral disease? How might you test for an elevated temperature?
Answers
Adults' armpit and vaginal hair likely serves the function of preventing friction and irritability during physical exertion.
Hooves, horns, and antlers are other portions of an animal's anatomy that must be made of material that is very similar to hair and nails.
Seasonal Affective illness (SAD) sufferers may find it easier to live in areas of the world with more daylight and longer daylight hours because these elements can lessen the symptoms of the illness.
It's crucial to use the ABCDE method while analyzing a spot on a friend's shoulder to check for the following indicators:
Asymmetry: Is the imperfection shaped in an unbalanced manner?Border: Are the blemish's margins ragged or poorly defined?Color: Is the blemish a unique color or does it have several colors?Diameter: Is the blemish larger than 6mm in diameter?Evolution: Has the blemish changed in size, shape, or color over time?Your acquaintance should visit a doctor if the blemish displays any of these symptoms since it may be an indication of skin cancer.
Infection, inflammation, or injury are just a few of the situations that can cause an elevated skin temperature.
Learn more about Seasonal Affective illness, here:
https://brainly.com/question/8018755
#SPJ1
At 275 °C a gas has a volume of 500 mL. What is the volume of this gas at 190°C?
Answers
Answer:
using the formula
v1/T1 =V2T2
make V2 subject of formula
V2= V1T2/T1
V2= 724mL
The volume of this gas at the 190°C will be 423 ml.
Explanation :We can resolve this issue by applying Charles' law. According to Charles' law, a gas's volume is directly inversely proportionate to its Kelvin temperature. To resolve this issue, we can apply the formula shown below:
[tex]\large{\implies{\bf{\boxed{\boxed{\dfrac{V1}{T1} = \dfrac{V2}{T2} }}}}}[/tex]
Where,
V1 is the gas's initial volume T1 is its starting temperature in Kelvin V2 is its finished volume T2 is its finished temperature in Kelvin.The temperatures must first be converted from Celsius to Kelvin. By raising each temperature by 273.15, we may achieve this.
Initial temperature (T1) is equal to 275 + 273 K.
500 mL is the initial volume (V1).
Final volume (V2) = Final temperature (T2) = 190 + 273.15 = 463.15 K Final temperature (T2) =?
V1/T1 = V2/T2
500/548.15 = V2/463.15
V2 = (500/548.15) * 463.15
V2 ≈ 423 mL
Therefore, at a temperature of 190°C, the volume of this gas would be approximately 423 mL.
Similar answers :
https://brainly.com/question/30911674
Help what's the answer?
Answers
The mass of the P4 that is reacted is 37.2 g
How does stoichiometry work?Stoichiometry works by using a balanced chemical equation to determine the mole ratio between reactants and products. This mole ratio is then used to convert the amount of one substance into the amount of another substance, using the mole concept and molar mass.
Using
PV = nRT
n = PV/RT
n = 1 * 39.6/0.082 * 298
n = 1.6 moles
From the reaction equation;
P4 + 6Cl2 → 4PCl3
1 mole of P4 reacts with 6 moles of Cl2
x moles of P4 reacts with 1.6 moles of Cl2
x = 1.6 * 1/6
= 0.3 moles
Mass of P4 = 0.3 * 124 g/mol
= 37.2 g
Learn more about stoichiometry:https://brainly.com/question/30215297
#SPJ1
what reactants are needed to produce this ester( shown above) through an acid catalyzed esterfication reaction? select one from each set( set a,b, and c).
Answers
The exact reactants needed to produce the ester in question, please provide the specific ester's chemical formula or name, along with the options available in each set (A, B, and C).
An ester can be formed by the reaction between a carboxylic acid and an alcohol in the presence of an acid catalyst, typically a strong acid such as sulfuric acid (H2SO4) or hydrochloric acid (HCl). The general reaction is as follows:
Carboxylic Acid (RCOOH) + Alcohol (R'OH) → Ester (RCOO-R') + Water (H2O)
In an acid-catalyzed esterification reaction, you need to select one carboxylic acid from set A and one alcohol from set B. The acid catalyst, which belongs to set C, will promote the reaction and facilitate the formation of the ester.
To learn more about : ester
https://brainly.com/question/28118164
#SPJ11
I need help please help me with these two questions (the second picture is in the comments)
Answers
sodium hydroxide
cobalt (II) phosphide
lead (IV) carbonate
Magnesium fluoride
lithium sulfite
ammonium phosphate
iron (II) oxide
calcium sulfate
silver nitride
sodium sulfide
rank each set of compounds from most acidic (i) to least acidic (iii): a) 2,4-dichlorobutyric acid i.) most b) 2,3-dichloro butyric acid ii.) c.) 3,3-dimethylbutryic acid iii.) least 3b. explain why you chose this order:
Answers
Answer:
Explanation:
i) Most acidic: 2,4-dichlorobutyric acid
ii) Intermediate acidity: 2,3-dichlorobutyric acid
iii) Least acidic: 3,3-dimethylbutyric acid
The acidity of a compound is determined by the stability of its conjugate base. A stronger acid will have a more stable conjugate base. In this case, the presence of electron-withdrawing groups like chlorine atoms in the carboxylic acid group increases the acidity of the compound by stabilizing the negative charge on the conjugate base.
Comparing the three compounds, 2,4-dichlorobutyric acid has two chlorine atoms which are more electronegative than the methyl groups present in the other compounds. The presence of these electron-withdrawing groups increases the acidity of the compound, making it the most acidic of the three.
2,3-dichlorobutyric acid has only one chlorine atom in the carboxylic acid group, making it less acidic than 2,4-dichlorobutyric acid but more acidic than 3,3-dimethylbutyric acid.
3,3-dimethylbutyric acid does not have any electron-withdrawing groups in the carboxylic acid group, making it the least acidic of the three compounds.
petrochemicals create the raw materials used to produce which of the following? pesticides plastics soaps computers all of these answer choices are correct.
Answers
Petrochemicals are used to create the raw materials used to produce all of the answer choices provided in the question, which includes pesticides, plastics, soaps, and computers. Petrochemicals are chemical compounds that are derived from petroleum or natural gas. These compounds are widely used in various industries to create the raw materials needed for the production of a wide range of products.
Pesticides are chemicals used to kill or control pests, and many of them are made from petrochemicals. Plastics are also made from petrochemicals and are used to make a variety of products such as packaging materials, toys, and automotive parts. Soaps are made from a combination of petrochemicals and natural oils, and they are used for personal hygiene and cleaning purposes. Petrochemicals are also used to create components of computers, such as circuit boards and other electronic parts.
In conclusion, petrochemicals are an essential component in the production of various consumer goods and industrial products, and they play a significant role in modern society.
For more such questions on Petrochemicals, visit:
brainly.com/question/28540307
#SPJ11
how did the salt concentration of each of the four buffer solutions (equilibration, binding, wash, and te) relate to its function?
Answers
The salt concentration of each of the four buffer solutions is given by the means of the function which is provided.
When an acid or a basic is supplied, buffers maintain a pH that is comparatively stable. As a result, they shield—or "buffer,"—other molecules in solution from the negative consequences of the extra acid or base. Buffers are vital for the correct operation of biological systems because they either contain a weak acid (HA) and its conjugate base (A), or a weak base (B) and its conjugate acid (BH+). In actuality, every biological fluid has a buffer to keep the pH at a healthy level.
Salinity (/slnti/), commonly known as saline water (also see soil salinity), is the degree of saltiness or quantity of salt dissolved in a body of water. The standard units of measurement are grammes of salt per litre (g/L) or grammes per kilogramme (g/kg; the latter is dimensionless and equal to ).
Salinity is a thermodynamic state variable that, along with temperature and pressure, controls physical properties like the density and heat capacity of the water. Salinity plays a significant role in defining many elements of the chemistry of natural waters and of biological activities within them.
Learn more about salt concentration:
https://brainly.com/question/14696785
#SPJ4
The salt concentration of each of the four buffer solutions (equilibration, binding, wash, and elution) plays a crucial role in their respective functions during protein purification.
1. Equilibration buffer: This buffer is used to prepare the column and adjust its conditions to match the sample's salt concentration. A moderate salt concentration helps maintain protein stability and prevents non-specific interactions.
2. Binding buffer: This buffer has a specific salt concentration to promote the target protein's binding to the resin, while minimizing non-specific binding of other proteins. The concentration ensures optimal interactions between the protein and the resin's functional groups.
3. Wash buffer: The salt concentration in the wash buffer is slightly higher than that in the binding buffer. This helps remove weakly bound and unbound contaminants, while keeping the target protein attached to the resin.
4. Elution buffer: The salt concentration in the elution buffer is the highest among the four solutions. This high salt concentration competes with the target protein for binding sites on the resin, causing the protein to be released from the column and collected in the eluate.
Overall, the varying salt concentrations in these buffers aid in the separation and purification of the target protein through a step-wise process.
To learn more about buffer click here
brainly.com/question/30546730
#SPJ11
a 40.0 ml sample of a 0.100 m aqueous nitrous acid solution is titrated with a 0.200 m aqueous sodium hydroxide solution. what is the ph after 10.0 ml of base have been added?
Answers
The pH of the solution after the addition of 10.0 mL of base is 3.35.
The balanced chemical equation for the reaction between nitrous acid and sodium hydroxide is:
HNO2 + NaOH → NaNO2 + H2O
Before any base is added, the nitrous acid solution is acidic, and so the pH is less than 7. The nitrous acid dissociates in water according to the following equilibrium:
HNO2 + H2O ⇌ H3O+ + NO2-
The equilibrium constant for this reaction is the acid dissociation constant, Ka, which is given by:
Ka = [H3O+][NO2-] / [HNO2]
At equilibrium, the concentration of nitrous acid that has dissociated is equal to the concentration of hydroxide ions that have been neutralized by the acid:
[HNO2] - [OH-] = [NO2-]
Initially, the concentration of nitrous acid in the solution is:
[HNO2] = 0.100 mol/L × 0.0400 L = 0.00400 mol
When 10.0 mL of 0.200 M sodium hydroxide solution is added, the number of moles of hydroxide ions added is:
[OH-] = 0.200 mol/L × 0.0100 L = 0.00200 mol
Using the stoichiometry of the balanced equation, the number of moles of nitrous acid that have reacted is also 0.00200 mol.
The concentration of nitrous acid remaining in the solution after the addition of base is:
[HNO2] = (0.00400 mol - 0.00200 mol) / 0.0500 L = 0.0400 mol/L
The concentration of nitrite ion in the solution is equal to the concentration of hydroxide ions that have been neutralized by the acid:
[NO2-] = [OH-] = 0.00200 mol / 0.0500 L = 0.0400 mol/L
The acid dissociation constant for nitrous acid is Ka = 4.5 × 10^-4 at 25°C.
Using the expression for the equilibrium constant, we can solve for the concentration of hydronium ions:
Ka = [H3O+][NO2-] / [HNO2]
[H3O+] = Ka × [HNO2] / [NO2-] = 4.5 × 10^-4 × 0.0400 mol/L / 0.0400 mol/L = 4.5 × 10^-4
Therefore, the pH of the solution after the addition of 10.0 mL of sodium hydroxide solution is:
pH = -log[H3O+] = -log(4.5 × 10^-4) = 3.35
So the pH of the solution after the addition of 10.0 mL of base is 3.35.
Click the below link, to learn more about Titration:
https://brainly.com/question/2728613
#SPJ11
Multiply. 15y^3/8ay x 2a/3y
Simplify your answer as much as possible
Answers
The simplified answer to the multiplication of the [tex]$\frac{15y^3}{8ay} \times \frac{2a}{3y}$[/tex] expression is [tex]$\frac{5y^2}{2a}$[/tex].
To multiply the given expression, we need to first simplify each fraction.
Starting with the first fraction:
[tex]$\frac{15y^3}{8ay}$[/tex]
We can simplify this fraction by canceling out the common factors in the numerator and denominator.
[tex]$\frac{15y^3}{8ay} = \frac{35yyy}{222ay}[/tex]
[tex]= \frac{35y^2}{22a}[/tex]
[tex]= \frac{15y^2}{4a}$[/tex]
Now we simplify the second fraction:
2a/3y
We can also simplify this fraction by canceling out the common factors in the numerator and denominator.
2a/3y = 2/(3y)
Now that we have simplified both fractions, we can multiply them together:
[tex]$\frac{15y^2}{4a} \times \frac{2}{3y}$[/tex]
Multiplying the numerators and denominators together gives:
[tex]$\frac{15y^2 \times 2}{4a \times 3y}[/tex]
[tex]= \frac{30y^2}{12ay}[/tex]
[tex]= \frac{5y^2}{2a}$[/tex]
To learn more about expression
https://brainly.com/question/14083225
#SPJ4
which control tube is used to compare to test broths 1, 2, and 3 in order to evaluate the effectiveness of the germicide?
Answers
The control tube that is used to compare to test broths 1, 2, and 3 in order to evaluate the effectiveness of the germicide is the positive control tube.
This control tube contains bacteria that are not exposed to the germicide and serves as a reference for the growth and viability of the bacteria in the absence of the germicide.
By comparing the growth and viability of the bacteria in the positive control tube to the growth and viability of the bacteria in the test broths, researchers can determine the effectiveness of the germicide in killing or inhibiting the growth of the bacteria.
It is important to use a positive control tube in order to establish a baseline for comparison and ensure accurate and reliable results
To learn more about : germicide
https://brainly.com/question/29316904
#SPJ11
A chemist that is involved in researching what reaction yields the most ethanol from crops is most likely considered to be working in the field of
Choose matching definition
Biochemistry
Pure Chemistry
Applied Chemistry
Albert Einstein
Answers
A chemist involved in researching the reaction that yields the most ethanol from crops is most likely considered to be working in the field of Applied Chemistry.
Applied Chemistry is a sub-discipline of chemistry that deals with the practical application of chemical principles and techniques to solve real-world problems. It involves the design, development, and optimization of chemical processes and products that are used in various industries such as agriculture, pharmaceuticals, energy, and materials science.
In this case, the chemist is applying their knowledge of chemical reactions and processes to optimize the production of ethanol from crops. This involves understanding the chemical composition of the crops, identifying the most efficient methods of converting them to ethanol, and optimizing the reaction conditions to maximize yield.
Biochemistry, on the other hand, is a sub-discipline of chemistry that focuses on the chemical processes and substances that occur within living organisms. Pure chemistry, also known as theoretical chemistry, is a sub-discipline of chemistry that is concerned with developing theories and models to explain chemical phenomena, without necessarily applying them to practical problems.
Albert Einstein, on the other hand, was a theoretical physicist who is widely regarded as one of the most influential scientists of the 20th century, known for his groundbreaking work on relativity and quantum mechanics.
Learn more about biochemistry here:
https://brainly.com/question/13132811
#SPJ11
what is the molarity of a solution prepared by dissolving 29.3 g kcl in water to a final volume of 500.0 ml?
Answers
The molarity of a solution prepared by dissolving 29.3 g KCl in water to a final volume of 500.0 ml is 0.786 M.
To calculate the molarity of a solution, follow these steps:
1. Determine the number of moles of solute (KCl) dissolved.
2. Convert the final volume of the solution to litres.
3. Calculate molarity using the formula: Molarity = moles of solute/volume of solution in litres.
Step 1: Determine the number of moles of KCl dissolved
- Molecular weight of KCl = 39.1 g/mol (K) + 35.45 g/mol (Cl) = 74.55 g/mol
- Moles of KCl = mass of KCl / molecular weight of KCl
- Moles of KCl = 29.3 g / 74.55 g/mol ≈ 0.393 moles
Step 2: Convert the final volume of the solution to litres
- Volume of solution = 500.0 mL = 500.0 / 1000 = 0.5 L
Step 3: Calculate the molarity
- Molarity = moles of solute/volume of solution in litres
- Molarity = 0.393 moles / 0.5 L ≈ 0.786 M
The molarity of the KCl solution is approximately 0.786 M.
To learn more about molarity, refer:-
https://brainly.com/question/8732513
#SPJ11
The molarity of the solution prepared by dissolving 29.3 g of KCl in water to a final volume of 500.0 ml is 0.786 M.
Explanation:
To find the molarity of the solution, first, calculate the number of moles of KCl in the solution.
The molecular weight of KCl is 74.55 g/mol [39.10 g/mol for potassium + 35.45 g/mol for chlorine].
Given the mass of KCl = 29.3 g
The number of moles of KCl is calculated by the formula:
moles of KCl = mass of KCl / molecular weight of KCl
moles of KCl = 29.3 g / 74.55 g/mol
moles of KCl = 0.393 moles
Molarity is defined as the number of moles of solute dissolved in 1 L of solution.
molarity of solution = moles of solute/volume of solution in liters
Therefore, the molarity of the solution can be calculated by:
molarity of solution = 0.393 moles / 0.5 L
Molarity of solution = 0.786 M
Therefore, the molarity of the solution prepared by dissolving 29.3 g of KCl in water to a final volume of 500.0 ml is 0.786 M.
To know more about molarity:
https://brainly.com/question/16587536
#SPJ11
Estimate the change in the thermal energy of water in a pond
a mass of 1,000 kg and a specific heat of 4,200 J/(kg. °C) if the
cools by 1°C.
er in a pond with
kg. "C) if the water
Answers
The change in the thermal energy of the water in the pond, a mass of 1,000 kg and the specific heat of 4,200 J/(kg. °C) is 4200 kJ.
The Mass of the water of the pond, m = 1,000 kg,
The specific heat of the water, C = 4,200 J/kg °C,
The change in temperature, ΔT = 1 °C,
The change in the thermal energy :
Q = mcΔT
where,
m = mass,
C = specific heat,
ΔT = change in temperature.
Q = 1000 × 4200 × 1
Q = 4200000 J
Q = 4200 kJ
The change in the thermal energy is 4200 kJ.
Thus, the change in thermal energy of the water in a pond is 4200 kJ.
To learn more specific heat here
https://brainly.com/question/29499912
#SPJ4
a 1.5 m solution of nacl has a volume of 0.534 l. if this is diluted to 0.80 m, what will be the final volume?
Answers
Dilution formula is: M conc . Vol conc = M diluted . Vol diluted
1.5 M . Vol conc = 0.80 M . 0.10L
Vol conc = 0.80 M . 0.10L / 1.5M = 0.053L
Does NaCl produce a solution?When water molecules push the ions apart, the ionic bond holding sodium and chloride ions together is destroyed. The water molecules surround the sodium and chloride atoms in this image after the salt compounds have been separated. The salt then starts to dissolve and turns into a homogeneous solution.
0.9% sodium chloride Injection, USP is a sterile, nonpyrogenic, isotonic sodium chloride and water solution for injection. Each mL of solution contains 9 mg of sodium chloride. It is only offered in single-dose vials and doesn't contain any bacteriostats, antibacterial agents, or extra buffers. Drugs for injection are diluted or dissolved using it.
learn more about water molecules
https://brainly.com/question/1313076
#SPJ1
a solution is made by dissolving 0.08100 moles of ba(oh)2 in enough water to make 820.0 ml of solution. what is the ph of the resulting solution?
Answers
The pH of the resulting solution is 12.70. This indicates that the solution is basic.
To find the pH of the resulting solution, we need to first determine the concentration of hydroxide ions (OH-) in the solution. First, we can calculate the molarity of the Ba(OH)2 solution by dividing the moles of Ba(OH)2 by the volume of the solution in liters:
0.08100 moles / 0.8200 L = 0.0988 M
Since each mole of Ba(OH)2 produces 2 moles of OH- ions in solution, the concentration of OH- ions in the solution is:
2 x 0.0988 M = 0.1976 M
To find the pH of the solution, we can use the equation:
pH = 14 - log[OH-]
Plugging in the OH- concentration we just calculated:
pH = 14 - log(0.1976)
pH = 12.70
Therefore, the pH of the resulting solution is 12.70. This indicates that the solution is basic, since a pH above 7 indicates basicity.
For more such questions on pH of the solution, visit:
brainly.com/question/30934747
#SPJ11