Answers
Answer:
A. Combustion.
Explanation:
Hello there!
In this case, according to the given information about the shown chemical reaction, whereby liquid pentane reacts with oxygen to produce carbon dioxide and hydrogen (should be water); we can use the attached file to realize this is a A. Combustion reaction as it has CO2 and H2O on the products side from a hydrocarbon fuel.
Regards!
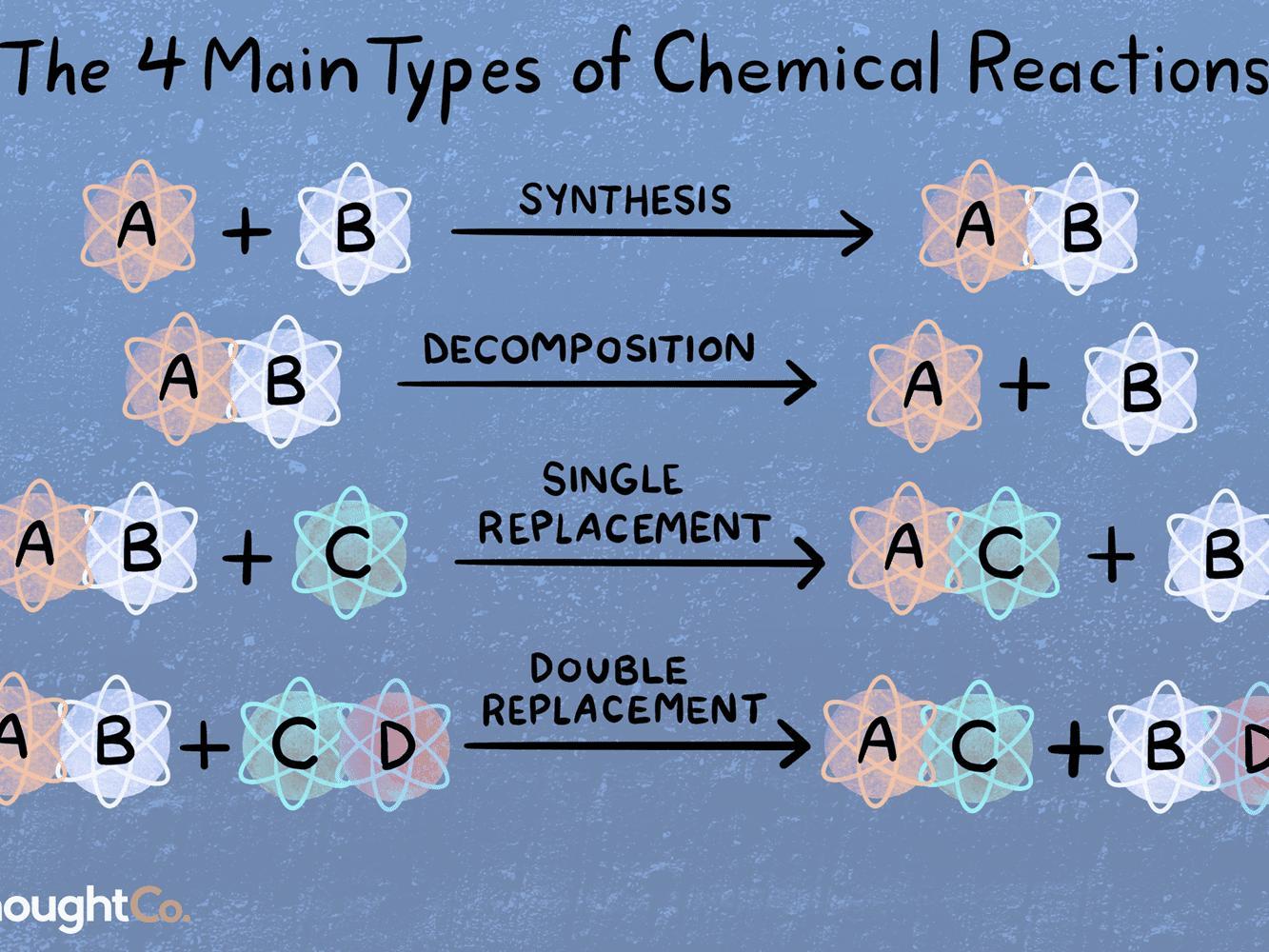
Related Questions
Which of the atoms shown has an atomic number of 4?
Answers
Answer:
B. Be
Explanation:
Question 23 (1 point)
Which of these bonds would have greatest ionic character
O-F
P.S
NE
Answers
Answer:
P.S
Explanation:
Because sulphur has low electronegative magnitude, hence low polarity potential.
The sulphur atom doesn't not get distorted hence increase in ionic degree character.
What Energy transformation occurs when gasoline burns in an automobile
Answers
Answer:
The release of energy from fuels is used to make other forms of energy. When gasoline burns in a car engine, some of the chemical energy in the gasoline is converted into heat. The heat is converted into mechanical energy. The mechanical energy moves the car.I hope this helped!
Explanation:
How do scientists design a system?
O A. They use a system that has already been designed.
O B. They ignore influences from sources outside of the experiment.
C. They include all possible influences in their model.
D. They isolate their experiment from unwanted influences.
Answers
Answer: D
Explanation:
The scientists design a system by isolate their experiment from unwanted influences. Therefore, option D is correct.
What is system ?Chemistry's field of systems chemistry strives to understand intricate webs of interdependent molecules and their system-level characteristics. These characteristics cannot be attributed to the individual components working independently, but rather to the aggregate behavior of the system's components.
A system is a well-organized group of components that work closely together to achieve a single objective. The system receives a variety of inputs, processes those inputs through certain steps to produce specific outputs, and then combines those outputs to achieve its overall objective.
The two main categories are natural systems and designed systems. Subatomic systems, various types of biological systems, our planet, the solar system, the galactic system, and the universe are all examples of natural systems.
Thus, option D is correct.
To learn more about the system, follow the link;
https://brainly.com/question/19843453
#SPJ2
Which of the following is true about the suns radiation?
40 percent is reflected by the atmosphere
It is long-wave
60 percent reaches the earths surface
It is short-wave
None of the rays are harmful
Answers
When considering free energy change, biochemists usually define a standard state, the biochemical standard state, which is modified from the chemical standard state to fit biochemical applications. Determine which of the phrases describe the biochemical standard state, the chemical standard state, or both.
a. Temperature is 25C
b. Initial concentration of reactants and products is 1M
Answers
Answer:
Chemical standard state
a. Temperature is 25C
b. Initial concentration of reactants and products is 1M
Biochemical standard state
a. Temperature is 25C
b. Initial concentration of reactants and products is 1M
Explanation:
The standard state is the reference state of a material which can be used to calculate its properties under other nonstandard conditions.
The biochemical standard state include;
a. Temperature is 25C
b. Initial concentration of reactants and products is 1M
Similarly, the chemical standard state include;
a. Temperature is 25C
b. Initial concentration of reactants and products is 1M
Hence the answer.
How many grams water will condense when 56,500 joules of energy is removed from steam at its boiling point
Answers
Answer:
Start your streak by answering any question. You'll get bonus points from day 2.
Question 4
2 pts
669.0 mL of oxygen are collected over water at 17.0 °C and a total
pressure of 785.0 mm of mercury. What is the volume (in mL) of dry
oxygen at 60.0 °C and 847.0 mmHg pressure?
Question 5
2 pts
Answers
Answer:
711.96 mL
Explanation:
Using the combined gas law equation;
P1V1/T1 = P2V2/T2
Where;
P1 = initial pressure (mmHg)
P2 = final pressure (mmHg)
V1 = initial volume (mL)
V2 = final volume (mL)
T1 = initial temperature (K)
T2 = final temperature (K)
According to the information provided in this question,
P1 = 785.0 mmHg
P2 = 847.0 mmHg
V1 = 669.0 mL
V2 = ?
T1 = 17.0 °C = 17 + 273 = 290K
T2 = 60.0 °C = 60 + 273 = 333K
Using P1V1/T1 = P2V2/T2
785 × 669/290 = 847 × V2/333
525165/290 = 847 V2/333
1810.91 = 2.54 V2
V2 = 1810.91 ÷ 2.54
V2 = 711.96 mL
The air we breathe contains different individual gases (mostly nitrogen and oxygen). Which of the following correctly describes the air we breathe? A. mixture B. liquid C. compound D. element
Answers
Answer:
A. Mixture
Explanation:
Our air has a group of gases. For example, you said nitrogen & oxygen, Which is significantly a mixture.
What is the limiting reactant when 1.50 g of lithium and 1.50 g of nitrogen combine to form lithium nitride, a
component of advanced batteries, according to the following unbalanced equation?
Li + N2 ⟶ Li3 N
Answers
Answer:
Li is limiting reactant
Explanation:
Based on the reaction:
6Li + N2 → 2Li3N
Where 6 moles of Li reacts per mole of N2
To solve this question we must convert the mass of each reactant to moles and using the chemical equation we can find limiting reactant
Moles Li -6.941g/mol-
1.50g * (1mol / 6.941g) = 0.2161 moles Li
Moles N2 -Molar mass:28g/mol-
1.50g * (1mol / 28g) = 0.0536 moles N2
For a complete reaction of 0.536 moles N2 are needed:
0.536 moles N2 * (6mol Li / 1mol N2) = 0.3214 moles Li
As there are just 0.2161 moles of Li
Li is limiting reactantThe addition of dimethylglycoxime, H2C4H6O2N2, to a solution containing nickel(II) ion gives rise to a precipitate: Ni2 2H2C4H6O2N2 Ni(H2C4H6O2N2)2 2H If 0.15 g nickel alloy is treated with dimethylglycoxime and .175 mg nickel dimethylglycoxime is collected. Determine the mass and percent of nickel in the alloy.
Answers
Solution :
The balanced equation is :
[tex]$Ni^{2+}+2H_2C_4H_6O_2N_2 \rightarrow Ni(H_2C_4H_6O_2N_2)_2+2H^+$[/tex]
Molar mass 56.7 116 290.7
From the balanced equation,
2 mole
= 2 x 116 g of [tex]$H_2C_4H_6O_2N_2$[/tex] produces 1 mole = 290.7 g of nickel dimethylglycoxime
or 2 x 116 mg of [tex]$H_2C_4H_6O_2N_2$[/tex] produces 1 mole = 290.7 g of nickel dimethylglycoxime
0.175 mg of [tex]$H_2C_4H_6O_2N_2$[/tex] produces [tex]$\frac{0.175 \times 290.7}{2 \times 116}$[/tex] = 0.219 mg of nickel dimethylglycoxime
290.7 g of [tex]$Ni(H_2C_4H_6O_2N_2)_2$[/tex] contains 58.7 mg of Ni
0.219 mg of [tex]$Ni(H_2C_4H_6O_2N_2)_2$[/tex] contains [tex]$\frac{0.219 \times 58.7}{290.7} = 0.0443$[/tex] mg of Ni
So mass of nickel, m = 0.0443 mg = [tex]$0.0443 \times 10^{-3}$[/tex] g
Percent of Nickel in the alloy = [tex]$\frac{\text{mass of nickel}}{\text{mass of alloy}} \times 100$[/tex]
[tex]$=\frac{0.0443 \times 10^{-3}}{0.159}\times 100$[/tex]
= 0.03%
Einstein's equation what does it mean? what does it apply ?
Answers
Einstein's Big Idea homepage. E = mc2. It's the world's most famous equation, but what does it really mean? "Energy equals mass times the speed of light squared." On the most basic level, the equation says that energy and mass (matter) are interchangeable; they are different forms of the same thing.
A student experimentally determines the density of a plastic cube using the caliper method, liquid displacement method, and suspension method. The cube's true density is 0.9822 g/cm3. Use the student's collected data below to answer the following questions.
Student's Collected Data
Cube's Mass 0.66g
Caliper Method
Edge Length 0.85 cm
Liquid Displacement Method
Volume of Liquid 5.5 mL
Volume of Liquid + Object 6.2 mL
Required:
Determine the density of the cube for each method.
Answers
Answer:
- density of the cube for Caliper Method is 1.08 g/mL
- density of the cube for Liquid Displacement Method is 0.94 g/mL
Explanation:
Given the data in the question;
The cube's true density = 0.9822 g/cm³
Student's Collected Data;
Cube's Mass = 0.66g
Caliper Method
Edge Length = 0.85 cm
Liquid Displacement Method
Volume of Liquid = 5.5 mL
Volume of Liquid + Object = 6.2 mL
For Caliper Method;
Edge Length = 0.85 cm
so the volume of the cube will be ( 0.85 cm )³ OR 0.614125 cm³ ≈ 0.61 cm³ = 0.61 mL
so Density will be;
Density[tex]_{caliper[/tex] = mass of cube / volume of cube
we substitute
Density[tex]_{caliper[/tex] = 0.66g / 0.61 mL
Density[tex]_{caliper[/tex] = 1.08 g/mL
Therefore, density of the cube for Caliper Method is 1.08 g/mL
For Liquid Displacement Method;
Volume of object = total volume - volume of liquid
= 6.2 mL - 5.5 mL = 0.7 mL
now, Density of object will be;
Density[tex]_{Liquid-Displacement[/tex] = mass / volume
we substitute
Density[tex]_{Liquid-Displacement[/tex] = 0.66 g / 0.7 mL
Density[tex]_{Liquid-Displacement[/tex] = 0.942857 ≈ 0.94 g/mL
Therefore, density of the cube for Liquid Displacement Method is 0.94 g/mL
A weather balloon calibrated at 0.00 degrees Celsius to have a volume of 20.0 L has what volume in L at -22.8 degrees Celsius pressure is held constant?
Answers
Answer:
New volume = 18.33 L
Explanation:
Given that,
Initial temperature, [tex]T_1=0^{\circ} C=273.15\ K[/tex]
Initial volume, [tex]V_1=20\ L[/tex]
Final temperature, [tex]T_2=-22.8^{\circ}C=250.35\ K[/tex]
We need to find the final volume when pressure is held constant. The relation between volume and temperature is given by :
[tex]V\propto T\\\\\dfrac{V_1}{V_2}=\dfrac{T_1}{T_2}\\\\V_2=\dfrac{V_1T_2}{T_1}\\\\V_2=\dfrac{20\times 250.35}{273.15}\\\\V_2=18.33\ L[/tex]
So, the new volume is equal to [tex]18.33\ L[/tex].
Increasing which factor will not increase the rate of a chemical reaction?
A: concentration of reactants
B: heat
C: product
D: surface area
Answers
Answer:
c. product
i had the test
please help with Chem I DON'T HAVE ENOUGH TIME!
if a 119g sample of water was allowed to evaporate completely, what volume of water vapour would be produced in milliliters?
Answers
As 1 L = 1000 g
so 119 grams = 0.119000 L
Hope it is helpful to u
If yes then plz mark me brainlest
We are given:
Mass of water: 119 grams
We know that one mole of a gas occupies 22.4L of volume
Number of moles of water:
Number of moles = given mass / Molar mass
Number of moles = 119 / 18 [molar mass of water = 18 grams/mol]
Number of moles = 6.61 moles
Volume occupied:
Volume = number of moles * 22.4 L
Volume = 6.61 * 22.4
Volume = 148L
Volume (in mL) = 1.48 * 10⁻¹ mL
ch3-co-ch2-ch2-ch3 IUpAC name
Answers
Answer:
2-pentanone.
Explanation:
Hello there!
In this case, for the given compound and, in agreement with the octet rule, it is possible to realize that the CO is actually C=O as shown below:
CH3 - C - CH2 - CH2 - CH3
||
O
Thus, since the C=O stands for the carbonyl group within the parent chain, we infer this is a ketone and more specifically 2-pentanone as it has five carbon atoms.
Regards!
3. At 34.0°C, the pressure inside a nitrogen-filled tennis ball with a volume of 148 cm3 is 212
kPa. How many moles of N2 are in the tennis ball?
Answers
Answer:
0.0123 mol
Explanation:
Step 1: Convert 34.0 °C to Kelvin
We will use the following expression.
K = °C + 273.15 = 34.0 + 273.15 = 307.2 K
Step 2: Convert 148 cm³ to L
We will use the conversion factors:
1 cm³ = 1 mL1 L = 1000 mL[tex]148cm^{3} \times \frac{1mL}{1cm^{3}} \times \frac{1L}{1000mL} = 0.148L[/tex]
Step 3: Convert 212 kPa to atm
We will use the conversion factor 1 atm = 101.325 kPa.
212 kPa × 1 atm / 101.325 kPa = 2.09 atm
Step 4: Calculate the moles of nitrogen gas
We will use the ideal gas equation.
P × V = n × R × T
n = P × V / R × T
n = 2.09 atm × 0.148 L / (0.0821 atm.L/mol.K) × 307.2 K = 0.0123 mol
The Properties of Liquids
Answers
Answer:
Properties of Liquids
Capillary Action. ...
Cohesive and Adhesive Forces. ...
Contact Angles. ...
Surface Tension. ...
Unusual Properties of Water. ...
Vapor Pressure. ...
Viscosity Viscosity is another type of bulk property defined as a liquid's resistance to flow. ...
Wetting Agents
Name: ___________________________ Date: __________ Period: ______ Solubility Rules Practice Worksheet Name or give the chemical formula for each of the following compounds. State whether they are soluble (will dissolve) or insoluble (will not dissolve) in solution. Use solubility rules. Chemical Formula Name Solubility NH4OH Ra(OH)2 Nickel (III) Acetate CsOH RbCl Potassium Phosphate MgS CaI2 Gold (II) Hydroxide Li3PO4 Platinum (II) Carbonate Barium Nitrate
Answers
Which of the following pairs of elements could NOT react to
form an ionic compound? Check all that apply.
Answers
Answer:
Carbon and Oxygen cannot react to form an ionic compound because the two elements are non-metals. To form an ionic bond, a metal combines with a non-metal through electrostatic attraction of oppositely charged ions.
Answer:
Explanation:
The two that won't are C and O. They will react, but not ionically. O is on the left of the Periodic table and C is more or less in the middle. They form CO carbon Monoxide and CO2 which is Carbon Dioxide. They are just not ionic.
Scientists recently discovered that firing low-energy particles at potatoes keeps the potatoes
from developing unwanted sprouts. They observed that these energy particles prevent cell
division in the sprouts. This stops the potatoes from sprouting for up to 120 days. How will this
development most likely affect the potato industry?
Answers
Answer:
By allowing the potatoes to be stored longer so they can be sold during winter months.
Explanation:
Since low energy particles are fired at the potatoes to prevent them from developing unwanted sprouts, it means that it prevents sprouts from growing on the potatoes.
Now, winter is wet period which encourages more growth of the sprouts. Thus, the way this development will affect the industry is that the potatoes will be stored for longer period and then sold in the winter.
CO
2 points
If there are 17.0 Liters of a gas in a balloon and the balloon expands to occupy a volume of 38.0 Liters with an initial pressure of 3.5 atm, what was the final pressure?
0.63 atm
Answers
What is the pOH of an aqueous solution with a pH of 10.6?
Answers
Answer:
pOH=3.4
Explanation:
pH + pOH = 14
So if you have the pH, rearrange the equation:
14-10.6=3.4
n today's experiment, Solutions A and B are prepared as follows. Solution A: Solution B: 2.0 mL of 3.00 x 10-4 M bromcresol green 2.0 mL of 3.00 x 10-4 M bromcresol green 5.0 mL of 1.60 M acetic acid (HAc) 2.0 mL of 0.160 M sodium acetate (NaAc) 2.0 mL of 0.200 M KCl diluted to a total volume of 50 mL diluted to a total volume of 50 mL How many mL of Solution A must be added to Solution B to give a buffer that is equimolar in HAc and Ac-
Answers
Answer:
2 mL of Solution A must be added to Solution B to give a buffer that is equimolar.
Explanation:
Given the data in the question;
First we determine the number of sodium acetate;
⇒ molarity × volume ( L )
⇒ 0.16 × 2.0 mL
⇒ 0.16 × 0.002 L
⇒ 0.00032
Now, Molarity of sodium acetate = moles / Volume(L)
⇒ ( 0.00032 / 50 ) × 1000
⇒ 0.0064
Since number of moles of acetic acid that should be added tp make equimolar solution is 0.00032
and Molarity of acetic acid is 0.16 molL⁻¹
Let X represent the volume that should be added.
so;
Molarity = Moles / Volume (L)
we substitute
0.16 = (0.00032 / X) × 1000
0.16 = 32 / X
X = 0.32 / 0.16
X = 2 mL
Therefore, 2 mL of Solution A must be added to Solution B to give a buffer that is equimolar.
2x²=8.pls help me i really need it
Answers
Explanation:
2x²=8
x²=8/2
x=√4
x=2
hope it helps.
Answer:
[tex]\huge \fbox \pink {A}\huge \fbox \green {n}\huge \fbox \blue {s}\huge \fbox \red {w}\huge \fbox \purple {e}\huge \fbox \orange {r}[/tex]
[tex] {2x}^{2} = 8 \\ {x}^{2} = \frac{8}{2} \\ {x}^{2} = 4 \\ x = \sqrt{4} \\ x = 2[/tex]
ʰᵒᵖᵉ ⁱᵗ ʰᵉˡᵖˢ
[tex] \huge\purple{ \mid{ \underline{ \overline{ \tt ꧁❣ ʀᴀɪɴʙᴏᴡˢᵃˡᵗ2²2² ࿐ }} \mid}}[/tex]
HELP NEEDED!!!!
The volume of a gas is 760 ml. The temperature changes from 20 oC to 40 oC. What will be the new volume?
A. 1520 ml
B. 1220 ml
C. 812 ml
D. 612 ml
Answers
Ethanol is a common laboratory solvent and has a density of 0.789 g/mL. What is the mass, in grams, of 131 mL of ethanol?
Answers
Answer:
m=103g
Explanation:
Hello there!
In this case, according to the given information, it is possible for us to say that this problem is solved by considering the concept of density, as the degree of compactness of a substance and mathematically defined as the mass divided by the volume (d=m/V). In such a way, for this problem, we solve for the mass as follows:
m=d*V
And we plug in the density and volume to obtain:
m=0.789g/mL*131mL
m=103g
Regards!
Ethanol is a common laboratory solvent and has a density of 0.789 g/ml. 103.359 g is the mass of 131 mL of ethanol.
Mass is a physical property of matter that measures the amount of substance in an object. It is typically measured in grams (g) or kilograms (kg).
To calculate the mass of 131 mL of ethanol, we need to use the density of ethanol.
Density of ethanol = 0.789 g/mL
Mass = Volume x Density
Substituting the given values, we get:
Mass = 131 mL x 0.789 g/ml
= 103.359 g
Therefore, the mass of 131 mL of ethanol is 103.359 g.
Learn more about mass here:
https://brainly.com/question/11954533
#SPJ6
The volume of a sample of carbon dioxide gas is 26.42 L at 73.0°C. What will its volume be at 92.0°C at constant pressure?
Answers
Answer:
[tex]V_2=27.87L[/tex]
Explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to solve this problem by using the Charles' law a directly proportional relationship to understand the volume-temperature behavior:
[tex]\frac{V_2}{T_2} =\frac{V_1}{T_1}[/tex]
Thus, we solve for the final volume, V2, and make sure the temperature are in Kelvin as shown below:
[tex]V_2 =\frac{V_1T_2}{T_1} \\\\V_2=\frac{26.42L(92+273.15)K}{(73+273.15)K} \\\\V_2=27.87L[/tex]
Regards!
Determine the net number of sigma bonds, the net number of pi bonds, and the overall bond order for N2+. Use 0.5 to indicate a fractional bond order.
Answers
Answer:
Net number of sigma bonds = 1
Net number of pi bonds = 2
Overall bond order = 3
Explanation:
Electronic configuration of N2 ia
1s2 2s2 2p3
There is head to head overlap in pz orbital. Thus, there is one sigma bond
Pi bond is formed whenever there is side wise overlapping. Since both px and py undergoes overlapping to form pi bond, there are two pi bonds
Bond order = 0.5 (bonding electron – antibonding electron)
= 0.5 (8-2) = 0.5*6 = 3
Answer:
Use 0.5 to indicate a fractional bond order.
σ bonds = 0.5
π bonds = 2
overall bond order = 2.5
Explanation:
trust me bro