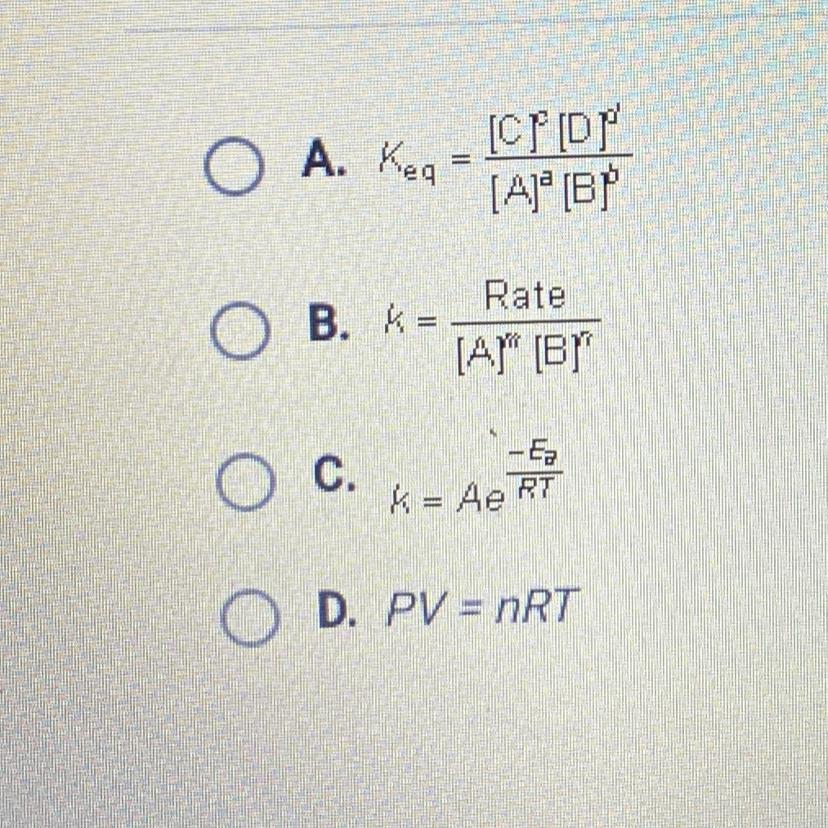
Which equation would be used to calculate the rate constant from initial
concentrations?
Answers
Answer: B
Explanation: I just did it
The equation for calculation of rate constant from initial concentration will be k= rate / [tex][A^{m}] [B^{n}][/tex]
What is rate constant?The proportionality variable in the expression represents the link between both the rate of a reaction as well as the concentrations of the reacting components often called the rate constant, sometimes specific rate constant.
What is equation?
A chemical reaction is represented by a word equation that uses the identities of the substance present.
The equation for calculation of rate constant from initial concentration will be k= rate / [tex][A^{m}] [B^{n}][/tex].
To know more about rate constant and equation.
https://brainly.com/question/20305871
#SPJ2
Related Questions
How many grams are there in 9.03 x 1023 molecules of CO2?
Answers
Answer:
The correct answer is - 66g.
Explanation:
Given:
molecules of CO2 = 9.03 x 10^23
We know:
1 mole of any substance = 6.02x10^23 molecules. (Avogadro's number)
M (CO2) = 12 + (2x16) = 12 + 32 = 44g
Solution:
The mass of CO2 with 9.03x10^23 molecules would be:
44g of CO2 = 6.02x10^23 molecules.
So, the mass in grams of CO2 (X) = 9.03x10^23 molecules
Xg of CO2 = (44x9.03x10^23)/6.02x10^23 = 66g
Thus, the correct answer would be - 66gm.
If the mass of all the reactants in a chemical reaction is 100g, what will the mass of all products be?
Answers
The energy transformation in an electromagnetic is from chemical to electrical to electromagnetic waves.
True or False?
Answers
I'LL GIVE 30 POINTS PLEASE ANSWER!!!
Which terms correctly identify the indicated structures in this sketch of a cell viewed under a microscope? Match each label to the correct cell part. Question 5 options: Golgi Apparatus Nucleus Endoplasmic Reticulum Mitochodrion Ribosome Lysosome
1.
2.
3.
4.
5.
6.
Answers
2. nucleus
3.Endoplasmic
4.Mitochondria
5.Ribosome
6.Lysosome
I need help! Please & Thank you!
Answers
Answer:
I think it is B
Explanation:
Select the chemical formula that contains 10 atoms of hydrogen.
2C4H10
2CH4
2C2H5
2C2H6
Answers
How many moles are in 281 g of Ca(OH)2?
Answers
Answer:
3.79 moles
Explanation:
To convert moles to gams of a substance we need to find the molar mass of the substance. For Ca(OH)₂ th molar mass is:
1Ca = 40.08g/mol
2O = 2*16g/mol = 32g/mol
2H = 2*1.01g/mol = 2.02g/mol
The molar mass is:
40.08g/mol + 32g/mol + 2.02g/mol = 74.1g/mol
And moles are:
281g * (1mol / 74.1g) =
3.79 molesHow many moles of gas are contained in a 50.0 L cylinder at a pressure of 100.0 atm and a temperature of 35.0°C?
Answers
Answer:
n = 2 moles (1 sig-fig)
Explanation:
Using the Ideal Gas Law equation (PV = nRT), solve for n (= moles) and substitute data for ...
pressure = P(atm) = 100atm
volume =V(liters) = 50L
gas constant = R = 0.08206L·atm/mol·K
temperature = T(Kelvin) = °C + 273 = (35 + 273)K = 308K
PV = nRT => n = PV/RT = (100atm)(50L)/(0.08206L·atm/mol·K)(308K)
∴ n(moles) = 1.978moles ≅ 2 moles gas (1 sig-fig) per volume data (= 50L) that has only 1 sig-fig. (Rule => for multiplication & division computations round final answer to the measured data having the least number of sig-figs).
Moles are the ratio of the mass and the molar mass of the substance. In a 50.0 L cylinder, 2 moles of gas are present at 100 atm and 35 degrees celsius.
What is an ideal gas equation?An ideal gas equation states the relationship between the moles of the substance, temperature, pressure, and volume. The ideal gas equation is given as,
[tex]\rm PV = nRT[/tex]
Given,
The pressure of the gas (P) = 100.0 atm
Volume of the gas (V) = 50.0 L
Temperature (T) = 308 K
Gas constant (R) = 0.08206 L atm/mol K
Substituting values in equation moles (n) is calculated as:
[tex]\begin{aligned} \rm n&= \rm \dfrac{PV}{RT}\\\\&= \dfrac{100 \times 50}{0.08206 \times 308}\\\\&= 1.978\end{aligned}[/tex]
Therefore, 1.978 or 2 moles of gas are present.
Learn more about moles here:
https://brainly.com/question/20343774
Calculate the percent by mass of benzene in a solution containing 14.2 g of benzene in 28.0 g of
carbon tetrachloride
Answers
Answer:
33.65%
Explanation:
total weight of solution=14.2+28=42.2g
benzene=14.2/42.2*100=33.65%
Please help me on this.
Answers
Answer: true
Explanation:
i hope this helps
Pleaseeee help pretty please
Answers
Answer:
A object made from iron .
Explanation:
what kind of chemical reaction is this?
Answers
What is the % of each element in (NH4)2(SO4)?
Answers
Nitrogen=21.1999%
Hydrogen=6.1023%
Sulfur=24.2660%
The percent composition of nitrogen, hydrogen, sulfur , oxygen in ammonium sulfate is 21.18%, 6.05%,24.21%,48.43% respectively.
What is percent composition?The percent composition is defined as a convenient way to record concentration of solution.It is a expression which relates solute to solvent as,mass of solute/mass of solution ×100.It helps in elemental analysis of a given compound by giving proportions in which they are present.
Percent composition of N= 28/132.14×100=21.18%
Percent composition of H=8/132.14×100=6.05%
Percent composition of S=32/132.14×100=24.21%
Percent composition of O=64/132.14×100=48.43%.
Thus, the percent composition of nitrogen, hydrogen, sulfur , oxygen in ammonium sulfate is 21.18%, 6.05%,24.21%,48.43% respectively.
Learn more about percent composition,here:
https://brainly.com/question/17505281
#SPJ2
How many particles are in one mole of copper (II) sulfate, CuSO4?
Answers
2
The current temperature, wind, precipitation and
other atmospheric conditions at any given time describes what?
A Controls
B Climate
C Tropics
D Weather
Answers
help plsssssssssssssssssssssss
Answers
Answer:
b chemical - thermal and mechanical
Explanation:
energy stored in food in is chemical, so it would only make sense of b) was the answer
A doctor tapes a patients knees with a hammer to test the reflex which can provide information about the condition of the _____
Answers
Answer:
lol this is what you get for not answering my question and stealing points
Explanation:
True or False: The reactants and the products of a chemical equation always have the same number of atoms.
Answers
Answer: True
Explanation: It always has the same number because the both have or are using the same product.
How many moles of O are in 12.6 mol Fe(NO3)3?
The answer is 113.4, rounded is 113
Explanation: Just multiply 12.6 mol Fe(NO3)3 by 9 ( 9 is the number of O atoms in the equation)
Answers
Answer: There are 113 moles of O in 12.6 moles of [tex]Fe(NO_3)_3[/tex]
Explanation:
Given : moles of [tex]Fe(NO_3)_3[/tex] = 113.4
To find : moles of O = ?
1 mole of [tex]Fe(NO_3)_3[/tex] contains = 9 moles of O atoms
Thus 12.6 mole of [tex]Fe(NO_3)_3[/tex] contains = [tex]\frac{9}{1}\times 12.6=113.4[/tex] moles of O atoms
Thus there are 113 moles of O in 12.6 moles of [tex]Fe(NO_3)_3[/tex]
Which reactions are oxidation-reduction reactions? Check all that apply.
(1) 2 upper N a plus upper C l subscript 2 right arrow 2 upper N a upper C l.
(2) Upper P b (upper O upper H) subscript 2 right arrow upper P b upper O plus upper H subscript 2 upper O.
(3)Upper C u plus 2 upper A g upper N upper O subscript 3 right arrow upper C u (upper N upper O subscript 3)subscript 2 plus 2 upper A g.
(4)Upper Z n upper B r subscript 2 plus 2 upper A g upper N upper O subscript 3 right arrow 2 upper A g upper B r plus upper Z n (upper N upper O subscript 3) subscript 2.
(5)Upper C upper H subscript 4 plus 2 upper O subscript 2 right arrow upper C upper O subscript 2 plus upper H subscript 2 upper O.
answer- 1,3, & 5
explanation- I did it on edge 2021
Answers
Answer:
the 1st 3rd and 5th ones
EDGE 2021
Explanation:
Answer:
A
C
E
Explanation:
Why is Newton's first law of motion is sometimes called the law of inertia
Answers
Answer:
The reason why Newtons first law of motion is sometimes called the law of inertia is because it states that if the object is in motion, it will not rest unless an unbalanced force acts on the object.
The genetic information found in DNA, chromosomes, and determines
A. the traits of the organism
B. the organism as an animal
C. the safety of the nucleus
D. the type of cell
Answers
Answer:
a
Explanation:
the traits of the organism
Enzymes in your body act as a catalyst. Thus the role of enzymes is to
O inhibit chemical reactions
o increase the rate of chemical reactions
O help you breathe
decrease the rate of chemical reactions
Answers
6. The Haber process for making ammonia (NH)
gas from its elements was developed by Fritz Haber
during WWI. Haber hoped to use ammonia as
fertilizer to grow food for Germany during the
Allie's blockade. How many liters of hydrogen
would be required to produce 40.0L of ammonia at
STP? N+H, NH,
Answers
Answer:
60 Liters
Explanation:
The equation for this reaction is given as;
N2 + 3H2 → 2NH3
From the reaction;
3 mol of H2 produces 2 mol of NH3
At STP;
1 mol = 22.4 L
This means
67.2 L ( 3 * 22.4) of H2 produces 44.8 L ( 2 * 22.4) of NH3
How many L of H2 would produce 40 L of NH3
67.2 = 44.8
x = 40
Solving for x;
x = 40 * 67.2 / 44.8
x = 60 L
HELP WITH A THESIS STATEMENT
I basically need a thesis statement on how DNA analysis relates to chemistry. The only problem is that I'm having trouble making it 'arguable'. My ideas were:
-DNA analysis relates to chemistry because it applies that knowledge when analyzing the DNA samples left at crime scenes in order to identify the suspect.
-DNA analysis relates to chemistry because it's a subcategory of chemistry, also known as forensic chemistry, where DNA left at crime scenes is analyzed to potentially link a suspect to a crime.
Do either of these sound good and are they arguable? If not can you reword it to be?
Answers
The second thesis statement is perfect. It supports the claim and presents main idea.
a 2.7 L of N2 is collected at 121kpa and 288 K . if the pressure increases to 202 kpa and the temperature rises to 303 K , what volume will the gas occupy?
Answers
Answer:
The gas will occupy a volume of 1.702 liters.
Explanation:
Let suppose that the gas behaves ideally. The equation of state for ideal gas is:
[tex]P\cdot V = n\cdot R_{u}\cdot T[/tex] (1)
Where:
[tex]P[/tex] - Pressure, measured in kilopascals.
[tex]V[/tex] - Volume, measured in liters.
[tex]n[/tex] - Molar quantity, measured in moles.
[tex]T[/tex] - Temperature, measured in Kelvin.
[tex]R_{u}[/tex] - Ideal gas constant, measured in kilopascal-liters per mole-Kelvin.
We can simplify the equation by constructing the following relationship:
[tex]\frac{P_{1}\cdot V_{1}}{T_{1}} = \frac{P_{2}\cdot V_{2}}{T_{2}}[/tex] (2)
Where:
[tex]P_{1}[/tex], [tex]P_{2}[/tex] - Initial and final pressure, measured in kilopascals.
[tex]V_{1}[/tex], [tex]V_{2}[/tex] - Initial and final volume, measured in liters.
[tex]T_{1}[/tex], [tex]T_{2}[/tex] - Initial and final temperature, measured in Kelvin.
If we know that [tex]P_{1} = 121\,kPa[/tex], [tex]P_{2} = 202\,kPa[/tex], [tex]V_{1} = 2.7\,L[/tex], [tex]T_{1} = 288\,K[/tex] and [tex]T_{2} = 303\,K[/tex], the final volume of the gas is:
[tex]V_{2} = \left(\frac{T_{2}}{T_{1}} \right)\cdot \left(\frac{P_{1}}{P_{2}} \right)\cdot V_{1}[/tex]
[tex]V_{2} = 1.702\,L[/tex]
The gas will occupy a volume of 1.702 liters.
To investigate the effect of salt water on a cell, a researcher places one cell sample in freshwater and a second cell sample in salt water. After six hours, the researcher observes and sketches the two cells as shown. What change in the cells occurred during the six hours the cells remained undisturbed? A.Osmosis occurred in the salt water cell sample because the water from inside the cell moved out of the cell to a lower concentration, causing the cell to shrink. B.Osmosis occurred in the freshwater cell sample because the water from outside the cell moved into the cell to a higher concentration, causing the cell to grow. C.Osmosis occurred in the salt water cell sample because the salt from outside the cell moved into the cell to a lower concentration, causing the cell to shrink. D.Osmosis occurred in the freshwater cell sample because the salt from inside the cell moved out of the cell to a higher concentration, causing the cell to grow.
Answers
Answer:
MARK ME BRAINLIEST
Explanation:
MARK ME BRAINLIEST
.When sodium atoms (Na) and chlorine atoms (Cl) join to make sodium chloride, or table salt, they form an ionic bond. Using this information, which pair of elements is most likely to form an ionic bond?
potassium (K) and fluorine (F)
aluminum (Al) and aluminum (Al)
sulfur (S) and oxygen (O)
calcium (Ca) and neon (Ne)
Answers
Answer:
potassium (K) and fluorine (F)
Explanation:
Potassium is an alkali metal just like sodium and fluorine is a halogen just like chlorine. A metal and nonmetal will often join to form an ionic compound.
Aluminum, if it was going to bond to itself, would be a metallic bond.
Sulfur and oxygen are both nonmetals and would form a covalent bond.
Calcium would not bond with neon as neon is a noble gas.
Answer:
A) potassium (K) and fluorine (F)
Explanation:
got it right on time for learning :)
If each image represents one mole of the substance pictured, which contains the greatest
amount of substance?
A)molecules
B) atoms
C)formula units
D)none; all amounts are the same
Answers
I searched it up
Mole measure the number of elementary entities of a given substance that are present in a given sample. Therefore, all amounts are the same. The correct option is option D.
What is mole?The SI unit of amount of substance in chemistry is mole. The mole is used to measure the quantity of amount of substance. It measure the number of elementary entities of a given substance that are present in a given sample. There are so many formula for calculating mole. We know one mole of any element contains 6.022×10²³ atoms which is also called Avogadro number.
Calculating the amount of a chemical in grams from moles is among the most often chemistry computations. You will employ the mole ratio of reactants to reagents when balancing equations.
Therefore, all amounts are the same. The correct option is option D.
.To know more about mole, here:
https://brainly.com/question/15209553
#SPJ2
Which system controls organs in times of stress?
Answers
Answer:
The autonomic nervous system has a direct role in physical response to stress and is divided into the sympathetic nervous system (SNS), and the parasympathetic nervous system (PNS). When the body is stressed, the SNS contributes to what is known as the “fight or flight” response.
Explanation: